Scleroderma, a complex autoimmune disease characterized by fibrosis of the skin and internal organs, presents significant challenges in management and treatment. While traditional therapies aim to manage symptoms and slow disease progression, emerging treatments offer promising avenues for more effective disease control. One such treatment modality gaining attention is bone marrow transplantation (BMT). This article delves into the potential of BMT as a management strategy for scleroderma, exploring its mechanisms, efficacy, and future prospects.
Understanding Scleroderma
What is Scleroderma?
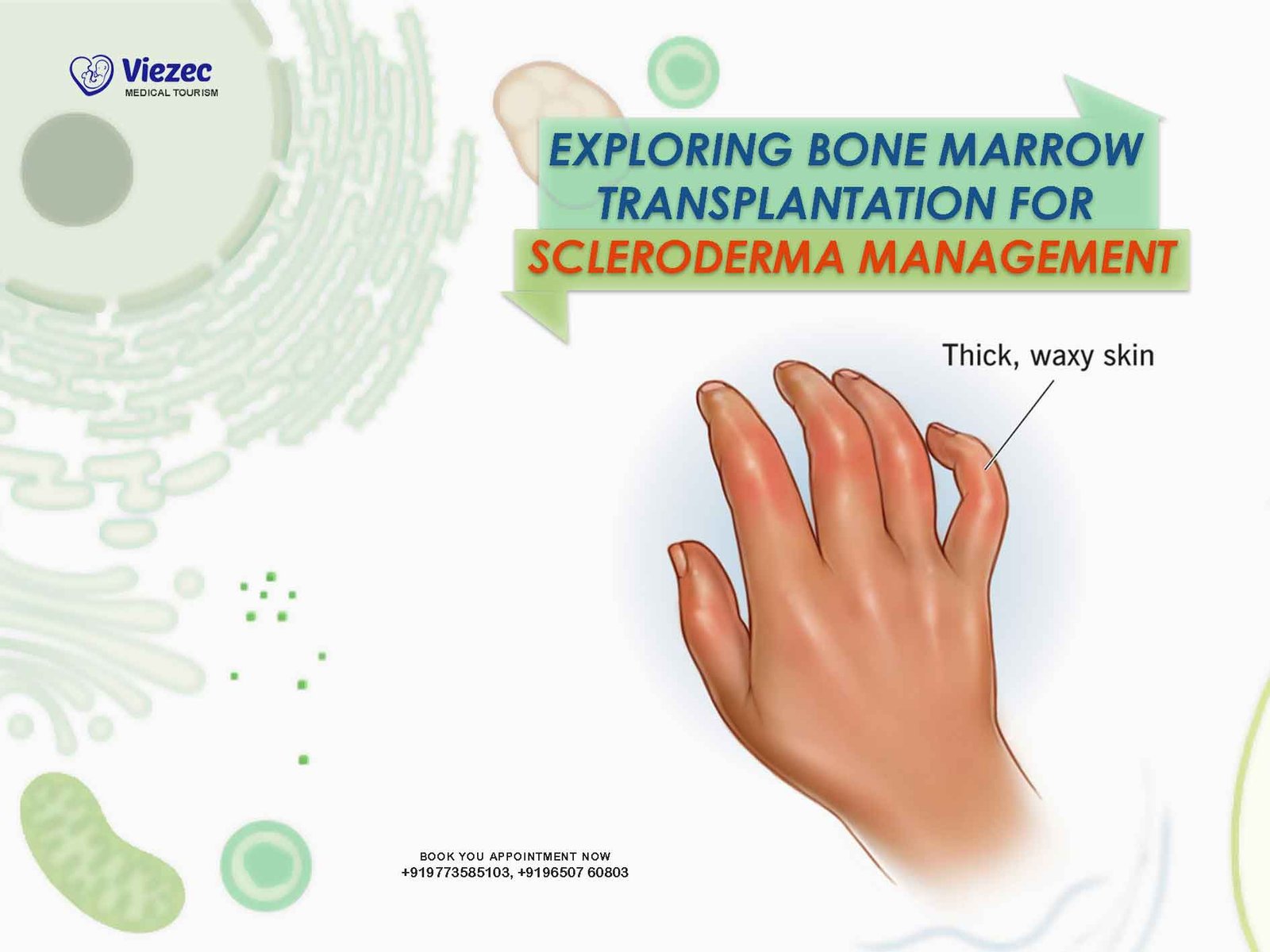
Scleroderma, derived from the Greek words “skleros” (hard) and “derma” (skin), is a chronic autoimmune disorder characterized by excessive collagen production leading to skin thickening and fibrosis of internal organs such as the lungs, heart, kidneys, and gastrointestinal tract. It falls under the umbrella of connective tissue diseases and manifests in two primary forms: localized and systemic scleroderma.
Pathogenesis of Scleroderma
The exact cause of scleroderma remains elusive, but it is believed to result from a combination of genetic predisposition, environmental factors, and dysregulation of the immune system. In scleroderma, immune cells and fibroblasts produce excessive amounts of collagen and other extracellular matrix proteins, leading to tissue fibrosis and vascular dysfunction. This dysregulated immune response ultimately contributes to the hallmark features of the disease.
Current Treatment Landscape
Challenges in Scleroderma Management
Managing scleroderma poses significant challenges due to its heterogeneous nature and variable clinical presentation. Treatment aims to alleviate symptoms, prevent organ damage, and improve quality of life, but options are limited and often provide only modest benefit. Conventional therapies include immunosuppressants, vasodilators, and drugs targeting specific symptoms such as pain and gastrointestinal dysfunction. However, these treatments are largely palliative and do not address the underlying immune dysregulation driving disease progression.
Role of Bone Marrow Transplantation
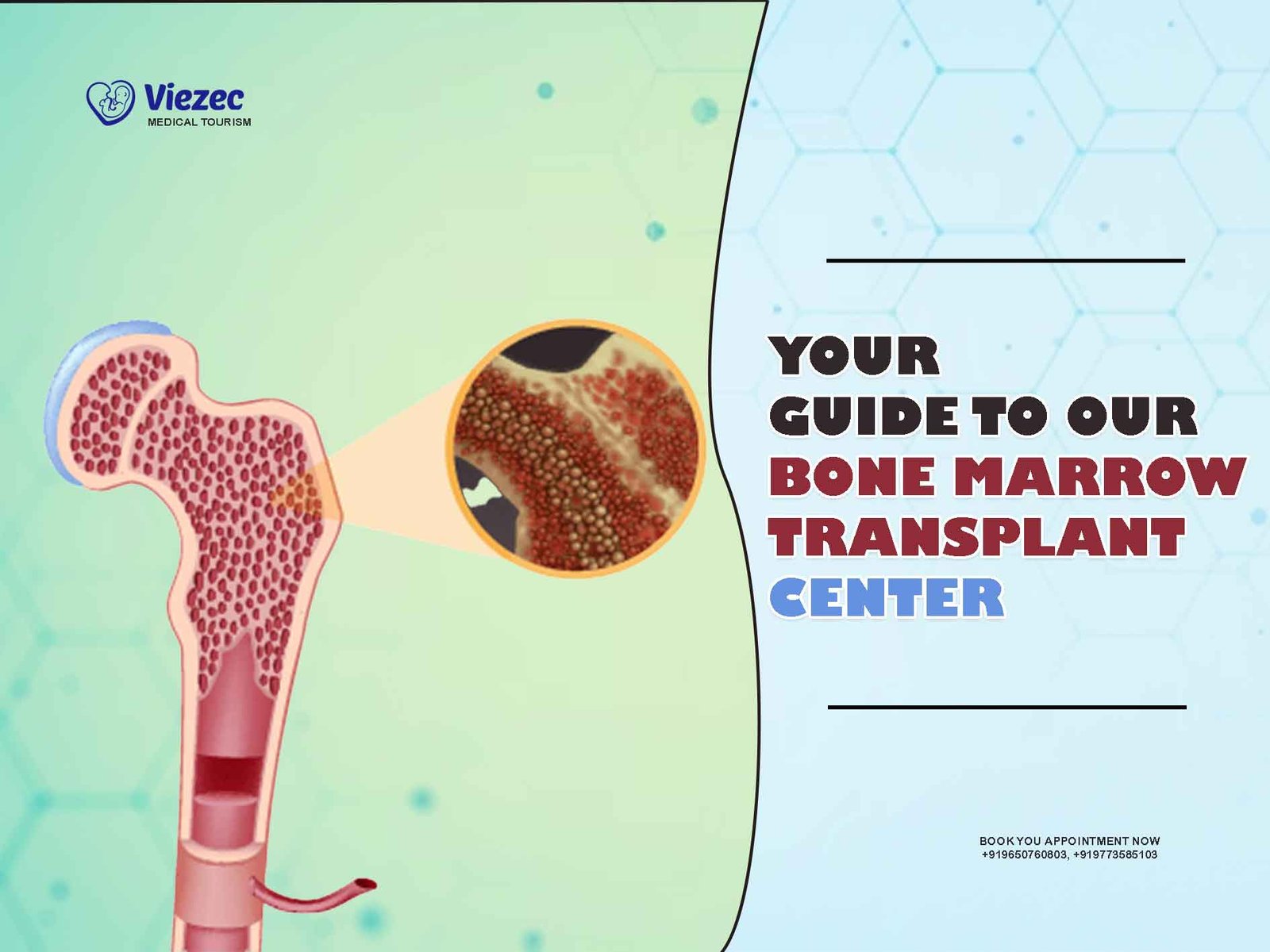
Amidst the limitations of conventional therapies, bone marrow transplantation (BMT) has emerged as a potential therapeutic approach for scleroderma. BMT, also known as hematopoietic stem cell transplantation (HSCT), involves the administration of high-dose chemotherapy to eradicate aberrant immune cells followed by infusion of autologous or allogeneic hematopoietic stem cells to restore immune function. While initially developed for hematologic malignancies, BMT has shown promise in treating autoimmune diseases by resetting the immune system and inducing immunological tolerance.
Mechanisms of Action
Immune System Reset
The rationale behind BMT in scleroderma lies in its ability to reset the immune system, effectively eliminating autoreactive immune cells responsible for the ongoing inflammatory response and tissue fibrosis. High-dose chemotherapy ablates the existing immune repertoire, including autoreactive T and B lymphocytes, while hematopoietic stem cell infusion facilitates immune reconstitution with a new, potentially tolerant immune system.
Induction of Immune Tolerance
BMT induces a state of immune tolerance wherein the newly regenerated immune cells exhibit reduced reactivity towards self-antigens, thereby mitigating autoimmune responses against host tissues. This tolerance-promoting effect is crucial in preventing disease relapse and maintaining long-term remission. Regulatory T cells (Tregs) play a pivotal role in mediating immune tolerance post-BMT by suppressing aberrant immune activation and promoting self-tolerance.
Clinical Evidence and Efficacy
Landmark Trials
Several clinical trials have investigated the efficacy of BMT in scleroderma, with notable studies demonstrating significant improvements in disease activity, skin fibrosis, and organ function following transplantation. The Autologous Stem Cell Transplantation International Scleroderma (ASTIS) trial, a landmark multicenter study, reported substantial clinical benefits in patients with early diffuse cutaneous scleroderma treated with autologous BMT compared to conventional therapy. Significant reductions in skin thickening, improvement in pulmonary function, and enhanced quality of life were observed in BMT recipients, underscoring its potential as a disease-modifying therapy.
Long-Term Outcomes
Long-term follow-up studies have further supported the durability of BMT responses in scleroderma, with sustained improvements in disease activity and organ involvement observed years after transplantation. The European Group for Blood and Marrow Transplantation (EBMT) registry reported favorable outcomes in a large cohort of scleroderma patients undergoing BMT, with durable remissions and low rates of disease relapse or progression. These findings highlight the long-term efficacy and safety of BMT as a therapeutic option for refractory scleroderma.
Safety Profile and Adverse Events
Treatment-Related Toxicities
Despite its potential benefits, BMT is associated with significant treatment-related toxicities, primarily stemming from the myeloablative conditioning regimen and immune suppression. Common adverse events include myelosuppression, infections, mucositis, and gastrointestinal complications, which can result in prolonged hospitalization and increased mortality risk. Careful patient selection, comprehensive pre-transplant evaluation, and stringent supportive care protocols are essential to minimize treatment-related complications and optimize transplant outcomes.
Infectious Complications
Immunosuppression following BMT predisposes patients to opportunistic infections, including bacterial, viral, and fungal pathogens. Prophylactic antimicrobial therapy, vigilant monitoring for signs of infection, and prompt intervention are critical in reducing infectious morbidity and mortality post-transplant. Strategies to mitigate infection risk include pre-transplant vaccination, antimicrobial prophylaxis, and judicious use of immunosuppressive agents to balance graft-versus-host disease (GVHD) prophylaxis with infection control.
Future Directions and Challenges
Personalized Medicine Approaches
The evolving landscape of precision medicine offers new avenues for tailoring treatment strategies in scleroderma based on individual patient characteristics, disease subtype, and immunological profile. Biomarker-guided approaches enable early identification of patients likely to benefit from BMT, optimizing patient selection and treatment outcomes. Integration of genomic, transcriptomic, and proteomic data may facilitate the development of predictive models for treatment response and disease prognosis, paving the way for personalized therapeutic interventions in scleroderma.
Novel Therapeutic Targets
Advances in our understanding of scleroderma pathogenesis have uncovered novel therapeutic targets implicated in immune dysregulation and fibrotic processes. Targeted biologic agents, such as monoclonal antibodies and small molecule inhibitors, hold promise in selectively modulating aberrant immune pathways and disrupting fibrotic cascades. Combination therapies leveraging synergistic mechanisms of action may enhance treatment efficacy and overcome therapeutic resistance, offering new hope for patients with refractory disease.
Translational Research Initiatives
Translational research efforts aimed at elucidating the underlying mechanisms of BMT-mediated immune reconstitution and tolerance induction are essential for optimizing transplant protocols and improving clinical outcomes. Preclinical models of scleroderma provide valuable insights into disease pathogenesis and therapeutic interventions, facilitating the development of innovative treatment strategies and biomarkers for clinical translation. Collaborative multicenter studies and patient registries serve as invaluable resources for evaluating long-term outcomes and refining transplant protocols in real-world settings.
Bone marrow transplantation represents a promising therapeutic approach for scleroderma management, offering the potential for disease modification and long-term remission.