Stem cell research stands at the forefront of medical innovation, promising revolutionary treatments and therapies for a myriad of diseases and conditions. However, its legal landscape is complex, shaped by ethical considerations, historical controversies, and evolving regulations. Understanding the legal framework surrounding stem cell research is crucial for both researchers and the public alike. This article provides an in-depth exploration of the legality of stem cell research, delving into its history, ethical dilemmas, international perspectives, regulatory bodies, clinical applications, and future directions.
Introduction to Stem Cell Research
Stem cells, with their unique ability to differentiate into various cell types, hold immense potential for regenerative medicine and tissue engineering. At the core of stem cell research is the quest to harness these cells to repair damaged tissues, organs, and systems within the human body. Stem cells can be broadly categorized into embryonic, adult, and induced pluripotent stem cells (iPSCs), each possessing distinct properties and applications. The importance of stem cell research transcends scientific curiosity, offering hope for patients suffering from debilitating conditions and diseases.
History of Stem Cell Legislation
The journey of stem cell legislation is fraught with challenges and controversies. Early regulations grappled with ethical concerns surrounding the use of embryonic stem cells, leading to landmark legal cases such as Roe v. Wade and Sherley v. Sebelius. These cases not only shaped the legal landscape but also ignited debates on the moral implications of stem cell research. Today, the legal framework governing stem cell research is a dynamic interplay of federal regulations, court rulings, and scientific advancements.
Ethical Considerations
The ethical dimensions of stem cell research have been a subject of intense scrutiny and debate. Central to this discourse is the controversy surrounding the use of embryonic stem cells, which raises profound questions about the beginning of life and the sanctity of human embryos. Religious perspectives further complicate the issue, with divergent views on the moral permissibility of manipulating human cells for research purposes. Public opinion and policy decisions reflect a delicate balance between scientific progress and ethical boundaries.
International Perspectives on Stem Cell Research
Stem cell research transcends national borders, prompting diverse approaches to regulation and oversight. A comparative analysis of global regulations reveals varying attitudes towards the use of stem cells in research and clinical practice. Notable legislation in regions such as the European Union, Japan, and China offers insights into different cultural and political contexts shaping stem cell policies. Harmonizing international standards remains a key challenge for the global scientific community.
Regulatory Bodies and Guidelines
In the United States, the Food and Drug Administration (FDA) plays a central role in regulating stem cell research and therapy. Its guidelines govern the development, testing, and marketing of stem cell-based products, ensuring safety and efficacy for patients. The National Institutes of Health (NIH) also provides essential guidance and funding support for stem cell research. Internationally, numerous stem cell societies and organizations collaborate to establish best practices and standards for ethical conduct and scientific rigor.
Clinical Applications and Trials
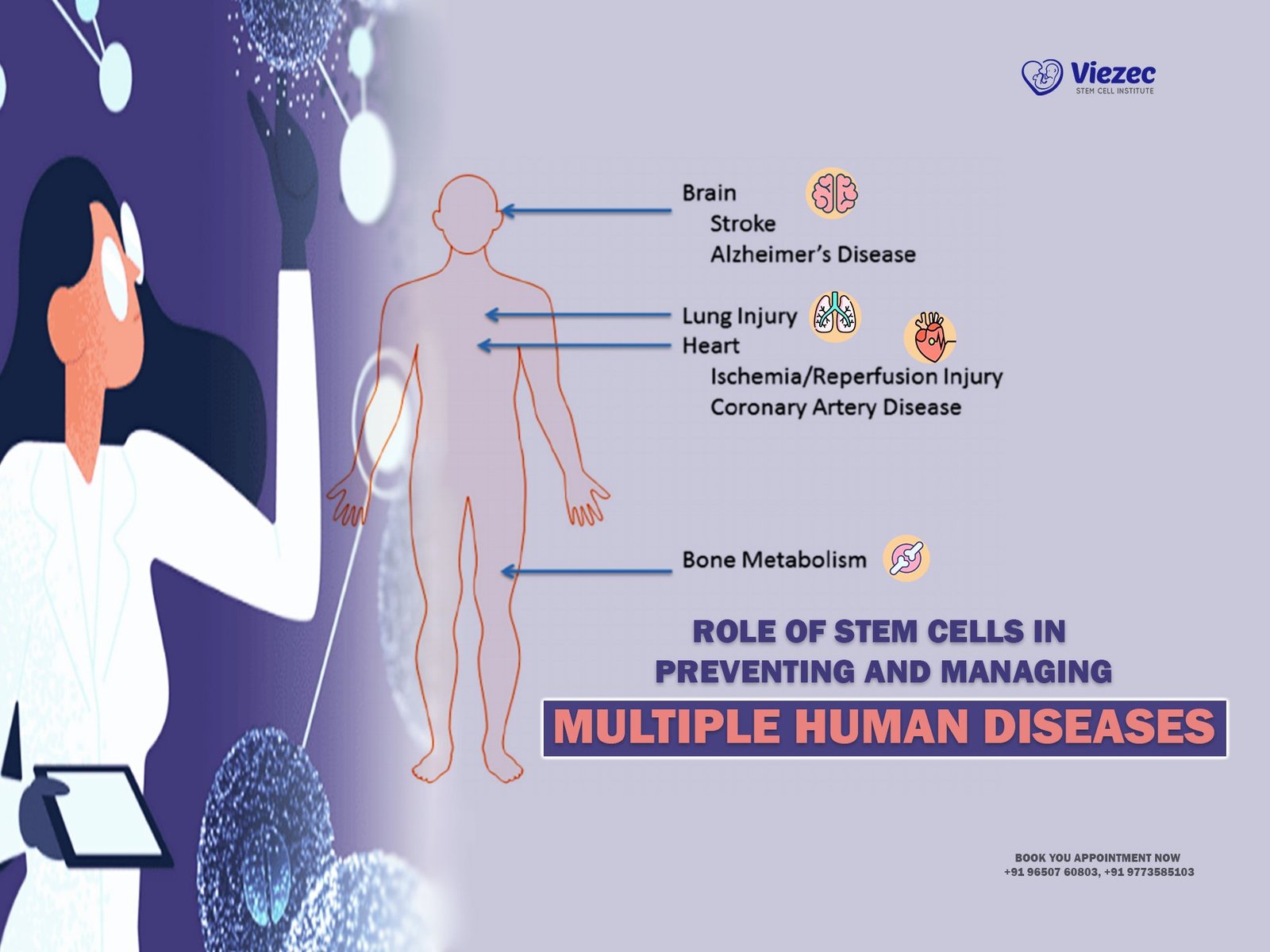
The translation of stem cell research into clinical applications holds immense promise for patients suffering from a wide range of ailments. Advancements in stem cell therapy have led to pioneering treatments for neurological disorders, cardiovascular diseases, and cancer. Current clinical trials offer hope for individuals seeking alternative therapies and interventions beyond conventional medicine. However, challenges such as patient safety, efficacy, and regulatory compliance remain paramount in the pursuit of innovative treatments.
Intellectual Property Rights
The intersection of stem cell research and intellectual property rights has spurred legal battles and controversies. Patents play a crucial role in protecting stem cell discoveries and incentivizing innovation in the field. However, disputes over patent ownership and licensing agreements have raised questions about access to essential technologies and the commercialization of stem cell therapies. Balancing intellectual property rights with the public interest poses significant challenges for policymakers and stakeholders.
Challenges and Controversies
Despite its potential, stem cell research faces numerous challenges and controversies. Safety concerns regarding the use of stem cell-based therapies underscore the need for rigorous preclinical and clinical testing. The potential for exploitation and oversight gaps raises ethical dilemmas surrounding informed consent, patient privacy, and research integrity. Moreover, the commercialization of stem cell therapies introduces complex ethical, legal, and economic considerations that require careful navigation.
Future Directions in Stem Cell Legislation
As technology advances and scientific understanding deepens, the landscape of stem cell legislation continues to evolve. Emerging technologies such as gene editing and tissue engineering present new opportunities and ethical dilemmas for policymakers and regulators. Potential legislative changes must balance scientific progress with ethical frameworks that safeguard human dignity and welfare. Collaborative efforts among stakeholders are essential to navigate the complexities of emerging technologies and shape future regulations.
Public Education and Engagement
Promoting public understanding and engagement with stem cell research is essential for informed decision-making and policy development. Addressing misconceptions and ethical concerns through education and outreach initiatives fosters trust and transparency in the scientific community. Involving the public in policy decisions ensures that diverse perspectives and values are considered in shaping the future of stem cell research. By fostering dialogue and collaboration, stakeholders can collectively address the complex ethical, legal, and social issues surrounding stem cell research.
Case Studies and Examples
Examining case studies and real-world examples provides valuable insights into the practical applications and ethical dilemmas of stem cell research. Success stories highlight the transformative potential of stem cell therapies in improving patient outcomes and quality of life. Ethical dilemmas encountered in clinical practice underscore the importance of robust regulatory frameworks and ethical oversight mechanisms. Lessons learned from past cases inform future policy decisions and research priorities, shaping the trajectory of stem cell research worldwide.
Collaboration and International Cooperation
In an increasingly interconnected world, collaboration and international cooperation are imperative for advancing stem cell research and addressing global health challenges. Joint research initiatives facilitate the sharing of resources, expertise, and knowledge across borders. Global efforts to harmonize regulations and standards promote scientific integrity and ensure equitable access to emerging therapies. By fostering a culture of collaboration, stakeholders can maximize the potential of stem cell research to improve human health and well-being on a global scale.
In conclusion, the legality of stem cell research is a multifaceted issue shaped by scientific, ethical, and regulatory considerations. Understanding the complex interplay of laws, policies, and societal values is essential for navigating the evolving landscape of stem cell research. By fostering dialogue, collaboration, and ethical engagement, stakeholders can harness the transformative potential of stem cell research while ensuring the responsible and equitable advancement of science for the benefit of humanity.