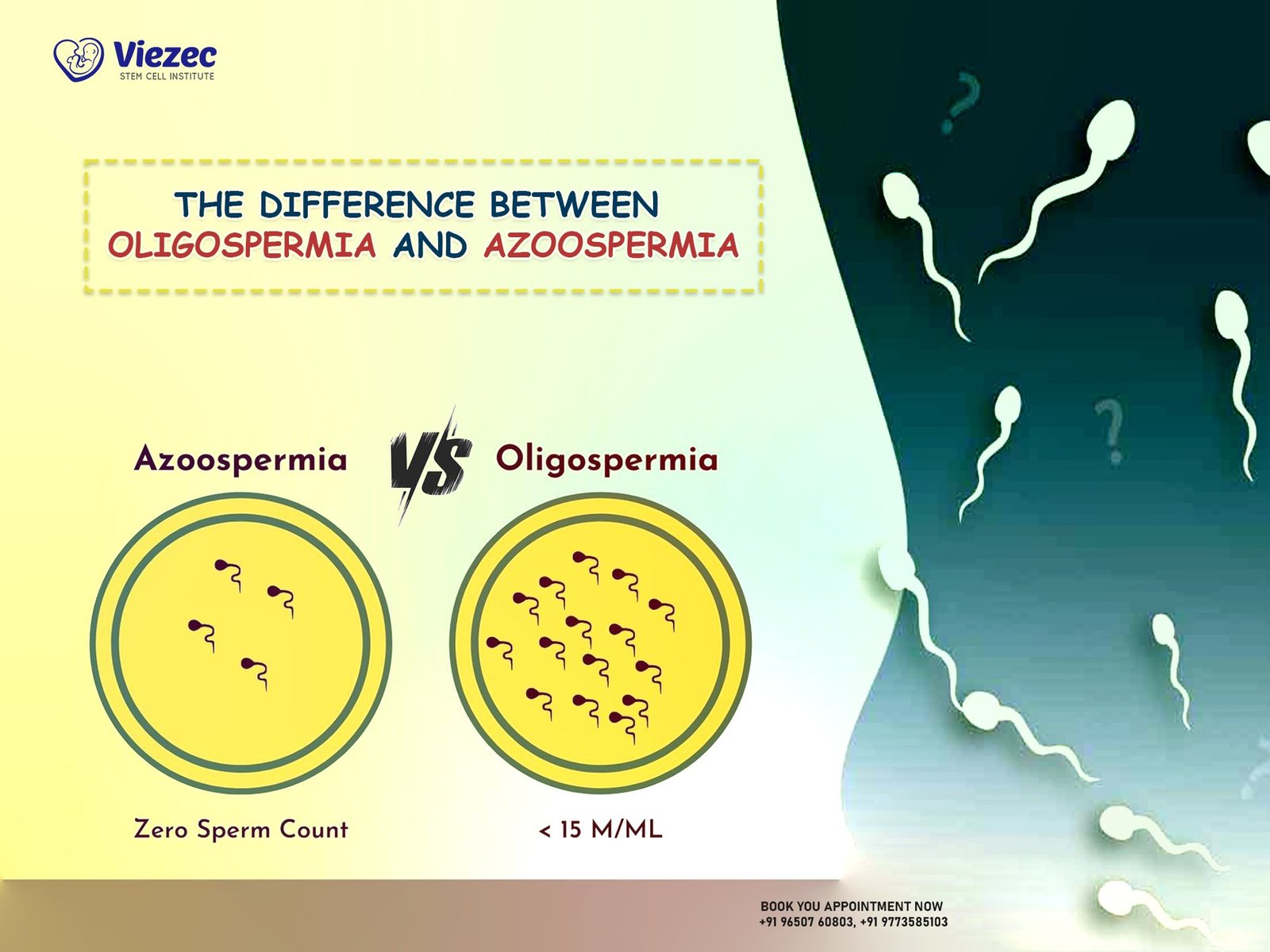
Multiple System Atrophy (MSA) stands as a complex and enigmatic neurodegenerative disorder that poses significant challenges to diagnosis and management. Initially identified as Shy-Drager syndrome in the early 1960s, MSA has evolved through various diagnostic criteria and classifications, reflecting the growing understanding of its distinct features and pathological underpinnings. Despite being relatively rare compared to other neurodegenerative diseases such as Parkinson’s or Alzheimer’s, MSA exacts a heavy toll on affected individuals, impairing their motor functions, autonomic regulation, and overall quality of life. In this article, we delve into the intricate facets of MSA, exploring its clinical presentation, underlying pathology, diagnostic dilemmas, and therapeutic strategies.
Understanding Multiple System Atrophy: Clinical Spectrum and Subtypes
Clinical Features
MSA manifests primarily as a combination of parkinsonian, autonomic, cerebellar, and pyramidal symptoms, leading to a heterogeneous clinical presentation. Patients typically exhibit progressive motor impairment, including bradykinesia, rigidity, and postural instability akin to Parkinson’s disease (PD). However, unlike PD, tremor is less prominent in MSA and often less responsive to dopaminergic therapy. Autonomic dysfunction is a hallmark feature, encompassing orthostatic hypotension, urinary incontinence, erectile dysfunction, and other dysautonomic symptoms, which can precede motor signs by years. Cerebellar involvement is evident through ataxia, dysarthria, and impaired coordination, while pyramidal features such as spasticity and hyperreflexia may also emerge. The combination of these motor, autonomic, and cerebellar symptoms delineates the clinical phenotype of MSA, contributing to its diagnostic complexity.
Subtypes of MSA
Recent research has highlighted the existence of two main subtypes of MSA based on predominant clinical features: MSA-P (predominantly parkinsonian) and MSA-C (predominantly cerebellar). MSA-P is characterized by prominent parkinsonian features, including bradykinesia, rigidity, and postural instability, akin to idiopathic PD. In contrast, MSA-C is distinguished by predominant cerebellar involvement, manifesting as ataxia, dysarthria, and other cerebellar signs. While these subtypes offer a framework for clinical categorization, considerable overlap exists between them, emphasizing the spectrum nature of MSA and the need for individualized patient evaluation.
Plan Your Stem Cell Therapy Procedure in India
Unraveling the Pathological Mechanisms of Multiple System Atrophy
Alpha-Synuclein Pathology
A defining pathological hallmark of MSA is the aberrant accumulation of alpha-synuclein protein within the central nervous system. Unlike in PD and other synucleinopathies, such as dementia with Lewy bodies (DLB), alpha-synuclein aggregates in MSA predominantly in oligodendrocytes rather than neurons, leading to the formation of glial cytoplasmic inclusions (GCIs). These GCIs are found throughout various regions of the brain, including the striatonigral and olivopontocerebellar pathways, corresponding to the motor and autonomic dysfunction observed in MSA. The propagation of alpha-synuclein pathology in MSA remains poorly understood but is believed to involve cell-to-cell transmission, potentially mediated by extracellular vesicles or other mechanisms.
Neuroinflammation and Glial Activation
In addition to alpha-synuclein pathology, MSA is associated with neuroinflammatory processes and reactive gliosis, implicating the involvement of the immune system in disease pathogenesis. Microglial activation and astrogliosis are evident in regions of alpha-synuclein deposition, suggesting a neuroinflammatory response to pathological insults. The precise interplay between neuroinflammation, alpha-synuclein aggregation, and neuronal injury in MSA is complex and multifaceted, representing an area of ongoing investigation. Targeting neuroinflammatory pathways may hold therapeutic promise in mitigating disease progression and associated neurodegeneration in MSA.
Diagnostic Challenges and Emerging Biomarkers
Clinical Diagnostic Criteria
Diagnosing MSA remains a clinical challenge, primarily due to its variable presentation and overlapping features with other neurodegenerative disorders, particularly PD and progressive supranuclear palsy (PSP). The current diagnostic criteria, established by the second consensus statement on MSA, emphasize the importance of identifying specific clinical features, such as autonomic dysfunction, cerebellar ataxia, and poor levodopa responsiveness, to differentiate MSA from its phenotypic mimics. However, achieving diagnostic certainty often necessitates longitudinal observation and integration of ancillary investigations.
Neuroimaging and Biomarkers
Advances in neuroimaging techniques, including structural and functional MRI, positron emission tomography (PET), and single-photon emission computed tomography (SPECT), have enabled the detection of characteristic patterns of neurodegeneration and neurotransmitter dysfunction in MSA. For instance, the “hot cross bun” sign on T2-weighted MRI, reflecting pontocerebellar atrophy, is considered highly specific for MSA. Moreover, PET and SPECT imaging can assess presynaptic dopaminergic function, aiding in the differentiation of MSA from PD. Beyond neuroimaging, efforts are underway to identify blood-based and cerebrospinal fluid (CSF) biomarkers indicative of alpha-synuclein pathology and disease severity in MSA, offering potential diagnostic and prognostic utility.
Therapeutic Strategies and Future Directions
Symptomatic Management
Currently, therapeutic options for MSA are limited, and disease-modifying treatments remain elusive. Symptomatic management aims to alleviate motor, autonomic, and other associated symptoms to improve patients’ quality of life. Pharmacological interventions may include levodopa and dopamine agonists for parkinsonian features, alpha-1 adrenergic agonists or fludrocortisone for orthostatic hypotension, and anticholinergic agents for urinary symptoms. However, response to these treatments is often variable and diminishes over time as the disease progresses, highlighting the need for alternative approaches.
Disease-Modifying Strategies
Efforts to develop disease-modifying therapies for MSA are underway, targeting various pathological mechanisms implicated in disease pathogenesis. These include strategies to reduce alpha-synuclein aggregation and propagation, mitigate neuroinflammation, enhance proteostasis and autophagy, and promote neuroprotection and neuronal repair. Clinical trials evaluating potential disease-modifying agents, such as alpha-synuclein antibodies, immunomodulatory agents, and neurotrophic factors, are ongoing, albeit in early stages. Additionally, non-pharmacological interventions, including physical therapy, speech therapy, and multidisciplinary care, play a crucial role in optimizing symptom management and maintaining functional independence in MSA patients.
Precision Medicine and Personalized Approaches
Given the clinical and pathological heterogeneity of MSA, there is growing recognition of the importance of personalized approaches to diagnosis and treatment. Precision medicine initiatives aim to characterize the molecular and genetic signatures of MSA subtypes and identify biomarkers predictive of disease progression and treatment response. Integrating multi-omics data, including genomics, transcriptomics, proteomics, and metabolomics, holds promise in elucidating the underlying molecular mechanisms driving MSA pathogenesis and identifying novel therapeutic targets. Furthermore, patient stratification based on clinical phenotypes, biomarker profiles, and genetic risk factors may enable tailored treatment strategies tailored to individual needs.
Collaborative Research and Translational Efforts
Addressing the complex challenges posed by MSA necessitates interdisciplinary collaboration and translational research efforts spanning basic science, clinical investigation, and therapeutic development. Establishing consortia and research networks dedicated to MSA, fostering data sharing and collaborative initiatives, and leveraging emerging technologies and platforms are essential steps toward accelerating progress in understanding and treating this devastating condition. Moreover, engaging patients and caregivers as partners in research and clinical trials is paramount to ensure that therapeutic development efforts align with patients’ priorities and unmet needs.
Contact us for free online appointment
Make a Decision
Multiple System Atrophy remains a formidable clinical entity, characterized by its multifaceted clinical presentation, underlying alpha-synuclein pathology, and diagnostic challenges. While significant strides have been made in elucidating the pathophysiological mechanisms of MSA and improving diagnostic accuracy, effective disease-modifying therapies remain elusive. Ongoing research endeavors, fueled by advances in neuroimaging, biomarker discovery, and precision medicine, hold promise for unraveling the enigma of MSA and transforming the landscape of therapeutic interventions. Through collaborative efforts and a patient-centered approach, we can strive to enhance the care and quality of life for individuals affected by this rare neurological condition.