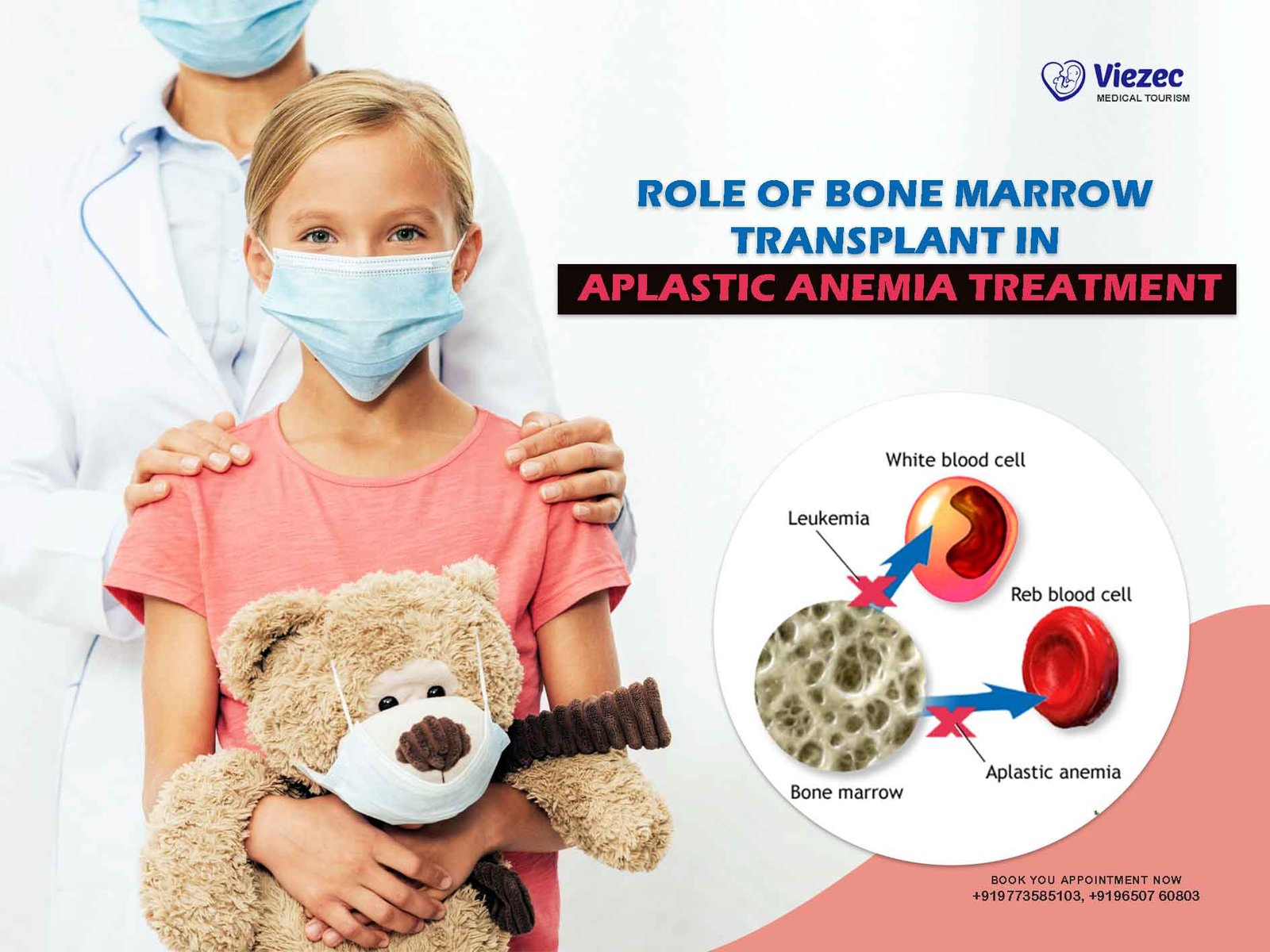
Aplastic anemia is a rare yet serious condition in which the bone marrow fails to produce sufficient blood cells, leading to fatigue, infections, and bleeding. This comprehensive guide explores the critical role of bone marrow transplant (BMT) as a potentially curative treatment, especially for patients with severe or very severe cases.
The article begins by breaking down the causes, risk factors, and symptoms of aplastic anemia, followed by an overview of diagnostic methods like blood tests and bone marrow biopsy. It details the severity levels of the disease and explains current treatment options, including immunosuppressive therapy, transfusions, and medications.
A large portion of the guide is dedicated to explaining when and how BMT is used, including types of transplants, donor matching, the step-by-step procedure, and post-transplant care. It also outlines success rates, potential complications like graft-versus-host disease, and the future of BMT through innovations such as reduced-intensity conditioning and gene editing.
Designed to inform and inspire, this article empowers patients, caregivers, and healthcare professionals with a hopeful, science-backed understanding of how BMT is transforming the prognosis of aplastic anemia.
Aplastic Anemia at a Glance
What Is Aplastic Anemia?
Aplastic anemia is a rare but life-threatening condition in which the bone marrow—the soft, spongy tissue inside your bones—stops producing enough new blood cells. This shortage affects all three major blood cell types: red cells (which carry oxygen), white cells (which fight infection), and platelets (which help with blood clotting).
As a result, patients may experience extreme fatigue, frequent infections, and unexplained bleeding or bruising. The condition can develop suddenly or gradually and often stems from the immune system mistakenly attacking the bone marrow, a phenomenon known as autoimmune-mediated aplastic anemia.
Other causes may include exposure to radiation, toxic chemicals (like benzene), certain medications (including chemotherapy drugs), and viral infections such as hepatitis or Epstein-Barr virus.
While it’s rare, early diagnosis and proper treatment are essential—because without enough blood cells, the body can’t function properly or protect itself from harm.
Key Causes and Risk Factors
Understanding the root causes of aplastic anemia is crucial for both prevention and early intervention. While the condition is rare, it can affect anyone—regardless of age or background. Several triggers may disrupt normal bone marrow function, either directly or by provoking an immune response against it.
Common Causes
-
Autoimmune Disorders: In many cases, the body’s own immune system mistakenly attacks the stem cells in the bone marrow, halting blood cell production.
-
Genetic Conditions: Inherited disorders like Fanconi anemia or Dyskeratosis congenita increase susceptibility, especially in children and young adults.
-
Toxic Chemical Exposure: Prolonged or intense exposure to industrial chemicals such as benzene (found in gasoline and some solvents) can impair marrow function.
-
Certain Medications: Chemotherapy drugs, some antibiotics, and antiepileptics have been linked to bone marrow suppression.
-
Radiation Exposure: High doses of radiation—either through cancer treatment or accidental exposure—can damage the bone marrow.
-
Viral Infections: Infections such as hepatitis, Epstein-Barr virus (EBV), cytomegalovirus (CMV), or HIV may trigger or worsen the condition.
Risk Factors to Keep in Mind
-
Family history of inherited bone marrow failure syndromes
-
Occupational hazards involving chemicals or radiation
-
History of autoimmune diseases like lupus or rheumatoid arthritis
-
Previous cancer treatments, particularly radiation or chemotherapy
Recognizing these factors can help identify high-risk individuals and guide timely testing or lifestyle changes to reduce exposure and risks.
Symptoms That Should Not Be Ignored
Aplastic anemia can develop gradually or strike suddenly—but in both cases, the symptoms often overlap with more common illnesses, making early diagnosis tricky. However, knowing what to watch for can be life-saving.
Key Warning Signs
-
Persistent Fatigue: One of the earliest and most noticeable symptoms. It’s more than just tiredness—it’s a deep, unrelenting exhaustion due to low red blood cell counts.
-
Frequent or Severe Infections: A low white blood cell count weakens the immune system, leaving patients vulnerable to frequent colds, fevers, or serious infections.
-
Easy Bruising or Bleeding: Even minor bumps can lead to large bruises, and cuts may bleed longer than normal. Nosebleeds, bleeding gums, or blood in urine/stool are also red flags.
-
Shortness of Breath: Reduced oxygen-carrying capacity in the blood can make even simple activities—like walking or climbing stairs—feel exhausting.
-
Pale or Sallow Skin: Low red blood cell levels can cause visible paleness or a yellowish skin tone.
When to Seek Medical Help
If you or someone you know experiences a combination of these symptoms—especially fatigue, bruising, and frequent infections—it’s important to seek medical evaluation promptly. A simple blood test could reveal the early signs of bone marrow failure, allowing for quicker diagnosis and more effective treatment.
How Aplastic Anemia Is Diagnosed
Diagnosing aplastic anemia requires a detailed look into the blood and bone marrow to confirm the extent and cause of the condition. Because the symptoms can mimic other illnesses, accurate testing is vital.
Common Diagnostic Tests
-
Complete Blood Count (CBC):
This is often the first test ordered. It typically reveals pancytopenia—a significant reduction in red blood cells, white blood cells, and platelets. -
Reticulocyte Count:
Reticulocytes are immature red blood cells. A low count indicates the bone marrow isn’t producing enough new red blood cells, which is characteristic of aplastic anemia. -
Liver and Kidney Function Tests:
These help rule out other causes of blood abnormalities and assess organ health before starting treatment. -
Viral Studies:
Tests for hepatitis viruses, Epstein-Barr virus (EBV), HIV, and other infections help determine if a virus might be the underlying cause.
Understanding Bone Marrow Biopsy
To confirm the diagnosis, a bone marrow biopsy is essential. During this procedure, a small sample of bone marrow is taken—typically from the hip bone—and examined under a microscope.
-
What Doctors Look For:
In aplastic anemia, the marrow appears hypocellular, meaning it has very few blood-forming stem cells and more fatty tissue than normal. -
Why It Matters:
The biopsy not only confirms aplastic anemia but also helps rule out other bone marrow diseases, like leukemia or myelodysplastic syndrome.
Severity Levels: Moderate vs. Severe
Aplastic anemia isn’t a one-size-fits-all diagnosis. Its severity determines the urgency and type of treatment needed. Doctors classify the condition into moderate, severe, and very severe categories based on blood counts and clinical symptoms.
Moderate Aplastic Anemia (MAA)
-
What it means:
Blood cell counts are reduced, but not to dangerously low levels. -
Symptoms:
May be mild or manageable at first. -
Treatment Approach:
Often monitored over time. Some patients may respond to supportive care or immunosuppressive therapy.
Severe Aplastic Anemia (SAA)
-
What it means:
Blood counts fall below critical thresholds. The bone marrow is mostly empty of stem cells. -
Symptoms:
Marked fatigue, recurrent infections, and serious bleeding episodes. -
Treatment Approach:
Urgent treatment is required—typically immunosuppressive therapy or bone marrow transplant (BMT) if a matched donor is available.
Very Severe Aplastic Anemia (VSAA)
-
What it means:
Extremely low neutrophil counts (below 200 cells per microliter), which puts patients at high risk of life-threatening infections. -
Symptoms:
Similar to SAA but more intense and rapid in progression. -
Treatment Approach:
Immediate and aggressive intervention, usually with BMT, especially in younger patients.
Current Treatment Options for Aplastic Anemia
Treatment for aplastic anemia depends on the severity of the disease, patient age, general health, and donor availability. The goal is to restore healthy blood cell production and prevent complications like infections or bleeding. For some, supportive care is enough in the short term, while others may need more aggressive treatments like a bone marrow transplant.
Immunosuppressive Therapy (IST)
For patients who don’t have a matched bone marrow donor—or are not candidates for transplant—immunosuppressive therapy is often the first line of treatment. This approach aims to quiet the immune system’s attack on the bone marrow.
-
Common Medications:
-
Antithymocyte globulin (ATG): A powerful agent that targets immune cells responsible for marrow damage.
-
Cyclosporine: Helps sustain the immune-suppressing effects of ATG and protect newly forming stem cells.
-
-
Who Benefits Most:
Typically used in older patients or those without a sibling donor. IST can lead to partial or even full remission, though relapses are possible.
Blood Transfusions and Medications
To manage symptoms and stabilize patients, supportive care is essential—especially during the early phase of the disease.
-
Red Blood Cell Transfusions: Help combat fatigue and shortness of breath.
-
Platelet Transfusions: Reduce the risk of spontaneous bleeding.
-
Growth Factors (e.g., G-CSF or EPO): May be used to stimulate the bone marrow to produce more white or red cells, especially in moderate cases.
These measures don’t cure the disease, but they buy time and improve quality of life while awaiting more definitive treatment.
When Is Bone Marrow Transplant Considered?
Bone marrow transplant (BMT)—also known as a stem cell transplant—is often the most definitive and potentially curative treatment for patients with severe or very severe aplastic anemia, particularly when a matched sibling donor is available.
Who Is a Good Candidate?
-
Younger patients (typically under age 40) with severe or very severe disease.
-
Patients with a fully matched sibling donor (MSD)—as outcomes are most successful with this match.
-
Those who do not respond to immunosuppressive therapy or experience a relapse.
Why BMT Offers a Cure
Unlike medications that manage symptoms or slow progression, BMT replaces the damaged marrow with healthy stem cells that can regenerate a fully functioning blood system. For eligible patients, this offers the best long-term survival and quality of life.
However, BMT isn’t without risks—it requires careful donor matching, conditioning (chemotherapy), and long-term follow-up. The decision to proceed is based on a thorough medical evaluation, balancing risk vs. reward based on individual patient profiles.
Bone Marrow Transplant Basics
Bone marrow transplant (BMT) is a complex but life-saving procedure designed to replace diseased or damaged bone marrow with healthy stem cells. These stem cells can come from a donor and have the remarkable ability to regenerate the body’s blood and immune systems.
What Happens During a BMT?
The transplant process involves several key steps:
-
High-Dose Conditioning:
Before receiving the transplant, the patient undergoes high-dose chemotherapy—and sometimes radiation—to destroy the faulty bone marrow and suppress the immune system, reducing the risk of rejection. -
Stem Cell Infusion:
Healthy donor stem cells are then infused intravenously, much like a blood transfusion. These cells travel to the bone marrow and begin creating new blood cells. -
Recovery & Engraftment:
Over the next 2 to 4 weeks, the infused stem cells start to “engraft”—that is, grow and multiply in the patient’s marrow, gradually restoring normal blood counts.
Types of Bone Marrow Transplants
Autologous vs. Allogeneic Transplants
-
Autologous Transplant:
Uses the patient’s own stem cells. Not used for aplastic anemia, since the marrow is already defective. -
Allogeneic Transplant:
Uses stem cells from a donor—which is the preferred and required option for treating aplastic anemia.
Matched Sibling and Unrelated Donors
-
Matched Sibling Donor (MSD):
Offers the best survival outcomes and lowest risk of complications like graft-versus-host disease (GVHD). -
Matched Unrelated Donor (MUD):
A strong alternative when a sibling match isn’t available. Outcomes have improved significantly thanks to better matching techniques and supportive care.
The BMT Procedure Step-by-Step
Undergoing a bone marrow transplant is a structured process that involves several carefully coordinated phases. Each stage is crucial to ensure the transplant’s success and minimize complications.
Pre-Transplant Evaluation
Before the procedure, patients go through a comprehensive evaluation to confirm readiness for transplant:
-
Medical Tests: Blood work, imaging scans, lung and heart function tests.
-
HLA Typing: To confirm compatibility with the donor.
-
Infection Screening: To address any infections before conditioning begins.
-
Psychological Counseling: Patients and families are offered emotional support and education to prepare for the journey ahead.
Stem Cell Collection and Conditioning
-
Donor Stem Cell Collection:
Donors give stem cells either from peripheral blood (most common) or directly from bone marrow. This part is painless for the recipient. -
Conditioning Therapy:
The patient receives high-dose chemotherapy, and sometimes radiation, to eliminate the defective marrow and suppress immune reactions.
Transplantation and Engraftment
-
Stem Cell Infusion:
The collected stem cells are transfused through an IV—similar to receiving a blood product. -
Engraftment:
Within 2–4 weeks, the stem cells begin producing healthy red cells, white cells, and platelets. During this time, patients are closely monitored for signs of recovery or complications.
Recovery and Post-Transplant Care
-
Infection Prevention:
Because immunity is very low during this phase, patients stay in a protected environment and receive antiviral, antibacterial, and antifungal medications. -
Ongoing Monitoring:
Frequent blood tests, marrow exams, and follow-up appointments help track engraftment, detect early signs of graft-versus-host disease, and assess overall recovery. -
Long-Term Care:
Recovery continues for months, and lifelong follow-up is essential. Survivors may need ongoing immune support, vaccinations, or management of late effects.
Donor Matching and Availability
A successful bone marrow transplant depends heavily on finding a donor whose immune system is compatible with the patient’s. This compatibility is determined by matching specific genetic markers known as human leukocyte antigens (HLA).
HLA Typing and Compatibility
-
What Is HLA?
HLA are proteins found on most cells in the body. A close HLA match between donor and recipient reduces the risk of the immune system rejecting the transplant or causing graft-versus-host disease (GVHD). -
How Matching Works:
Doctors perform detailed HLA typing—typically looking at 8 to 10 key markers. A matched sibling usually has a 25% chance of being fully compatible. If no sibling is available, registries are searched for matched unrelated donors (MUDs).
Role of Global Donor Registries
When a sibling match isn’t available, international registries can offer a lifeline:
-
Be The Match® (U.S.)
-
World Marrow Donor Association (WMDA)
-
DKMS (Germany, India, and other countries)
These databases connect patients with millions of potential unrelated donors worldwide. Thanks to global collaboration, many patients—especially from underrepresented ethnic groups—now have better chances of finding a suitable donor.
Umbilical Cord Blood as an Alternative
In some cases, umbilical cord blood—collected at birth and stored in public banks—can be used:
-
Pros:
-
Easier to match (less strict HLA compatibility needed)
-
Readily available
-
Lower risk of GVHD
-
-
Cons:
-
Limited stem cell quantity (better for children and small adults)
-
Slower engraftment time, which increases infection risk during recovery
-
Cord blood is particularly useful when no matched adult donor is available, offering hope for patients with rare HLA types or urgent needs.
Success Rates and Prognosis
The outlook for patients with aplastic anemia has improved significantly in recent decades, thanks to advances in bone marrow transplantation, better donor matching, and supportive care. Still, outcomes vary based on several individual and clinical factors.
Factors That Affect Treatment Outcome
-
Age of the Patient:
Younger patients, especially those under 40, tend to recover more successfully due to stronger immune function and fewer comorbidities. -
HLA Match Level:
A full HLA match with a sibling or unrelated donor significantly improves the chances of a successful transplant. -
Time From Diagnosis to Transplant:
Early transplants—ideally within the first few months after diagnosis—often lead to better results. -
Pre-Transplant Health:
Patients without prior infections, multiple transfusions, or prolonged marrow failure generally fare better.
Long-Term Survival and Quality of Life
-
Survival Rates:
With a matched sibling donor, 5-year survival rates exceed 80–90% in younger patients. Matched unrelated donor (MUD) transplants have also shown strong outcomes, though slightly lower. -
Quality of Life:
Many patients return to school, work, and family life post-transplant. Some may need to manage long-term effects such as fatigue or fertility issues, but the majority experience significant improvement in overall health.
Success in Pediatric vs. Adult Patients
Children often show higher success rates and faster recovery due to:
-
Lower risk of chronic conditions
-
Fewer prior transfusions or infections
-
More responsive immune systems
Adults can also do well, especially with reduced-intensity conditioning regimens and improved supportive care—but their prognosis may vary more depending on age and overall health.
Potential Risks and Complications
While bone marrow transplant (BMT) offers a potential cure for aplastic anemia, it also comes with significant risks. These complications are closely monitored and managed by the care team throughout the transplant process and beyond.
Graft-versus-Host Disease (GVHD)
GVHD is one of the most serious complications of allogeneic transplants, where the donor’s immune cells attack the recipient’s tissues.
-
Types of GVHD:
-
Acute GVHD: Occurs within the first 100 days post-transplant. Affects skin, liver, and gastrointestinal tract.
-
Chronic GVHD: Develops later and may impact multiple organs, causing symptoms similar to autoimmune diseases.
-
-
Management:
Treated with immunosuppressive drugs like steroids, tacrolimus, or cyclosporine. Early detection and personalized care help minimize severity.
Infection and Immune Suppression
Because high-dose chemotherapy and the transplant process weaken the immune system, patients are at elevated risk for:
-
Bacterial infections (e.g., sepsis, pneumonia)
-
Viral reactivations (e.g., cytomegalovirus or herpes simplex)
-
Fungal infections (e.g., candidiasis or aspergillosis)
Preventive strategies include:
-
Sterile environments during hospitalization
-
Prophylactic antiviral, antibacterial, and antifungal medications
-
Strict hygiene practices and frequent health monitoring
Monitoring and Managing Side Effects
Beyond GVHD and infections, patients may experience:
-
Short-term side effects: Nausea, vomiting, hair loss, mouth sores (mucositis), and fatigue.
-
Long-term effects: Fertility challenges, thyroid issues, bone density loss, or organ damage (liver, heart, or lungs) in rare cases.
Lifelong follow-up care is essential to detect and manage these late effects, ensuring patients continue to thrive after recovery.
Future of BMT in Aplastic Anemia
The landscape of bone marrow transplantation is evolving rapidly. Ongoing innovations aim to improve outcomes, reduce complications, and expand access for more patients—especially those who previously lacked suitable donor matches or were considered high-risk.
Innovations in Conditioning Protocols
Traditional conditioning regimens—while effective—can be harsh, particularly for older patients or those with pre-existing health issues.
-
Reduced-Intensity Conditioning (RIC):
Uses lower doses of chemotherapy to prepare the body for transplant, making BMT safer for older adults and medically vulnerable patients.-
Benefits: Fewer side effects, shorter hospital stays, and improved tolerability without compromising effectiveness in many cases.
-
Emerging Role of Gene and Cellular Therapies
While BMT remains the gold standard, gene therapy and cellular engineering are reshaping the future:
-
Gene Editing:
Technologies like CRISPR are being explored to correct faulty genes directly in the patient’s own stem cells—potentially eliminating the need for a donor altogether. -
Induced Pluripotent Stem Cells (iPSCs):
Scientists are developing personalized stem cells from the patient’s own tissue, which could one day provide custom, rejection-free marrow replacements.
Personalized Treatment Approaches
Thanks to advancements in genomics and precision medicine, doctors can now tailor treatment plans based on each patient’s:
-
Genetic makeup
-
Immune system profile
-
Response to previous therapies
This individualized approach ensures that patients receive the most effective—and least risky—treatment available.