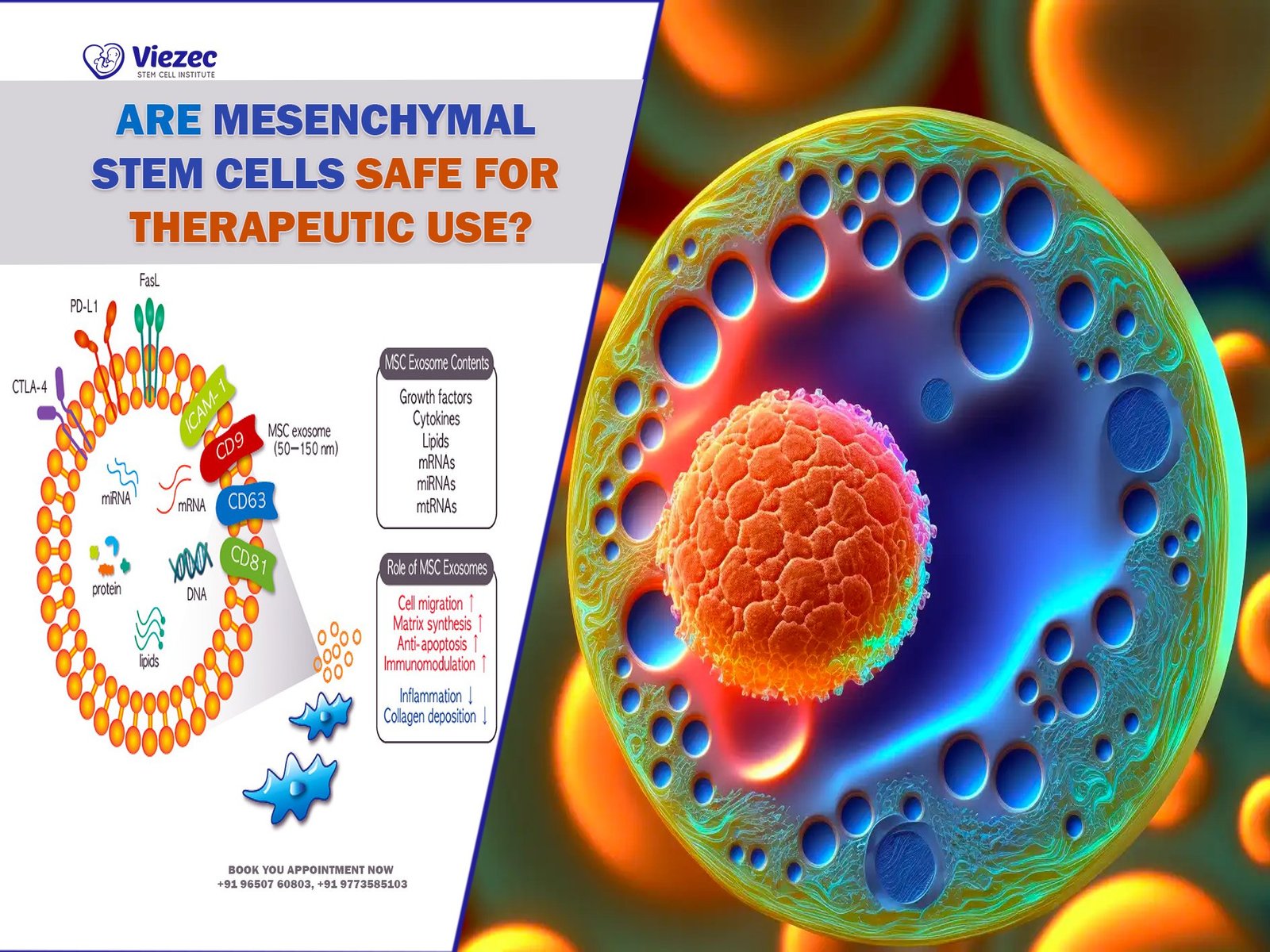
Wharton’s Jelly, a gelatinous substance found in the umbilical cord, has gained significant attention in regenerative medicine due to its abundance in mesenchymal stem cells (MSCs). Unlike other sources of MSCs, such as bone marrow or adipose tissue, Wharton’s Jelly is readily accessible, non-invasive to obtain, and does not raise ethical concerns.
Mesenchymal Stem Cells: Definition and Characteristics
Mesenchymal stem cells (MSCs) are multipotent stromal cells capable of self-renewal and differentiation into various cell types, including osteoblasts, chondrocytes, and adipocytes. Their immunomodulatory properties and regenerative potential make them attractive candidates for therapeutic applications in various medical conditions.
Rationale for Investigating Therapeutic Potential
The unique properties of Wharton’s Jelly MSCs, including their abundance, immunomodulatory effects, and low immunogenicity, underscore their potential as a promising therapeutic tool for a wide range of diseases and disorders.
Isolation and Characterization
Techniques for Isolating Wharton’s Jelly Mesenchymal Stem Cells
Several methods, such as enzymatic digestion or explant culture, are employed to isolate MSCs from Wharton’s Jelly. Each technique has its advantages and limitations, influencing the yield and quality of the obtained cells.
Phenotypic and Functional Characterization Methods
Characterizing Wharton’s Jelly MSCs involves assessing their surface marker expression, differentiation potential, and immunomodulatory properties through flow cytometry, immunocytochemistry, and functional assays.
Comparative Analysis with Other Mesenchymal Stem Cells
Comparative studies between Wharton’s Jelly MSCs and MSCs derived from other sources reveal unique characteristics, including higher proliferation rates and superior immunomodulatory effects, making them a preferred choice for therapeutic applications.
Immunomodulatory Properties
Mechanisms of Immunomodulation
Wharton’s Jelly MSCs exert their immunomodulatory effects through various mechanisms, such as cytokine secretion, cell-cell contact, and induction of regulatory T cells, dampening inflammatory responses and promoting tissue repair.
Impact on Inflammatory Diseases
Preclinical and clinical studies demonstrate the efficacy of Wharton’s Jelly MSCs in mitigating inflammatory conditions, including rheumatoid arthritis, inflammatory bowel disease, and graft-versus-host disease, through their anti-inflammatory and immunosuppressive actions.
Potential in Autoimmune Disorders
The immunomodulatory properties of Wharton’s Jelly MSCs hold promise for the treatment of autoimmune disorders, such as multiple sclerosis and lupus, by restoring immune homeostasis and preventing aberrant immune responses.
Regenerative Medicine Applications
Role in Tissue Repair and Regeneration
Wharton’s Jelly MSCs facilitate tissue repair and regeneration by promoting angiogenesis, enhancing cell proliferation, and modulating the inflammatory microenvironment, offering potential therapeutic interventions for wound healing and tissue regeneration.
Targeted Therapies for Musculoskeletal Disorders
The regenerative potential of Wharton’s Jelly MSCs makes them a promising candidate for treating musculoskeletal disorders, including osteoarthritis and bone fractures, by promoting cartilage repair and bone regeneration.
Advancements in Wound Healing
Clinical studies investigating the use of Wharton’s Jelly MSCs in chronic wound management demonstrate accelerated wound healing, reduced inflammation, and improved tissue regeneration, highlighting their therapeutic efficacy in non-healing wounds.
Neuroprotective Effects
Crossing the Blood-Brain Barrier: Challenges and Solutions
The ability of Wharton’s Jelly MSCs to cross the blood-brain barrier is critical for their therapeutic efficacy in neurological disorders. Innovative delivery strategies, such as intrathecal administration and nanoparticle-based carriers, offer promising solutions to enhance CNS targeting.
Neuroregeneration and Neuroplasticity Enhancement
Wharton’s Jelly MSCs promote neuroregeneration and neuroplasticity by secreting neurotrophic factors, modulating inflammatory responses, and enhancing synaptic connectivity, offering potential treatments for neurodegenerative diseases and traumatic brain injuries.
Clinical Trials and Potential Neurological Applications
Ongoing clinical trials investigating the safety and efficacy of Wharton’s Jelly MSCs in neurological disorders, including Parkinson’s disease and spinal cord injury, provide valuable insights into their therapeutic potential and pave the way for future clinical applications.
Cardioprotective Potential
Cardiac Tissue Regeneration Mechanisms
Wharton’s Jelly MSCs contribute to cardiac tissue repair and regeneration by differentiating into cardiomyocytes, promoting angiogenesis, and inhibiting fibrosis, offering promising strategies for treating ischemic heart disease and heart failure.
Treatment Strategies for Ischemic Heart Disease
Preclinical studies demonstrate the efficacy of Wharton’s Jelly MSCs in improving cardiac function, reducing infarct size, and enhancing neovascularization in ischemic heart disease models, highlighting their potential as a cell-based therapy for myocardial repair.
Future Directions in Cardiac Therapy
Exploring novel delivery methods, optimizing cell dosing and timing, and enhancing cell survival and engraftment are essential considerations for maximizing the therapeutic benefits of Wharton’s Jelly MSCs in cardiac regeneration and heart repair.
Anti-cancer Properties
Tumor Microenvironment Modulation
Wharton’s Jelly MSCs exhibit anti-cancer properties by modulating the tumor microenvironment, suppressing tumor growth, and enhancing anti-tumor immune responses, offering potential synergistic approaches for cancer therapy.
Potential for Targeted Cancer Therapy
The ability of Wharton’s Jelly MSCs to home to tumor sites and deliver therapeutic payloads, such as cytotoxic agents or oncolytic viruses, presents a promising strategy for targeted cancer therapy with reduced systemic toxicity.
Challenges and Opportunities in Oncology
Addressing concerns regarding the potential pro-tumorigenic effects of MSCs, optimizing the timing and dosage of MSC-based therapies, and enhancing their tumor-targeting specificity are critical challenges that need to be addressed to harness the full potential of Wharton’s Jelly MSCs in cancer treatment.
Clinical Trials and Translational Research
Overview of Completed and Ongoing Trials
A growing number of clinical trials are investigating the safety and efficacy of Wharton’s Jelly MSCs in various medical conditions, including neurological disorders, cardiovascular diseases, and autoimmune disorders, providing valuable insights into their therapeutic potential and safety profile.
Safety and Efficacy Considerations
Ensuring the safety and efficacy of Wharton’s Jelly MSC-based therapies requires rigorous preclinical evaluation, standardized manufacturing processes, long-term follow-up studies, and close monitoring of adverse events in clinical trials.
Regulatory Landscape and Future Prospects
Navigating regulatory frameworks, obtaining regulatory approvals, and addressing ethical and legal considerations are essential steps in advancing Wharton’s Jelly MSC-based therapies from bench to bedside, paving the way for their widespread clinical adoption.
Optimizing Therapeutic Delivery
Routes of Administration
The versatility of WJ-MSCs lies in the myriad ways they can be administered for therapeutic effect. Intravenous infusion, direct injection, and tissue engineering are among the primary routes explored for their delivery. Each method offers distinct advantages and challenges, influencing the choice of administration based on the targeted condition and desired outcome.
Scaffold-Based Strategies
Scaffold-based approaches represent a promising avenue for enhancing the efficacy of WJ-MSC therapy. By providing a supportive framework for cell growth and proliferation, scaffolds facilitate the targeted delivery of stem cells to damaged tissues, optimizing their regenerative potential. This technique holds particular promise in tissue engineering applications, where the precise spatial organization of cells is crucial for successful tissue regeneration.
Bioengineering Approaches for Enhanced Efficacy
Bioengineering strategies offer innovative solutions to enhance the therapeutic efficacy of WJ-MSCs. From genetic modification to preconditioning techniques, bioengineering enables the customization of stem cells to suit specific therapeutic objectives. By enhancing their survival, proliferation, and differentiation capabilities, bioengineered WJ-MSCs hold immense potential for addressing a wide range of medical conditions with greater precision and efficiency.
Ethical and Regulatory Considerations
Ethical Issues Surrounding Stem Cell Research
Despite their therapeutic promise, stem cell research raises complex ethical concerns regarding the source of cells and the implications of their use. Ethical debates surrounding the derivation of WJ-MSCs from umbilical cords highlight the need for careful consideration of moral principles and societal values in scientific endeavors. Balancing scientific advancement with ethical responsibility remains paramount in navigating the ethical landscape of stem cell research.
Regulatory Frameworks and Guidelines
The regulatory landscape governing the use of WJ-MSCs is shaped by stringent guidelines aimed at ensuring patient safety and ethical standards. Regulatory bodies play a crucial role in overseeing the development, testing, and clinical application of stem cell therapies, enforcing compliance with established protocols and standards. Adherence to regulatory frameworks is essential to mitigate potential risks and safeguard the integrity of stem cell-based interventions.
Patient Consent and Privacy Concerns
Informed consent and privacy protection are fundamental principles guiding the ethical practice of stem cell therapy. Patients must be fully informed about the nature of the treatment, potential risks, and alternatives before consenting to participate in clinical trials or procedures involving WJ-MSCs. Additionally, measures to safeguard patient privacy and confidentiality must be implemented to uphold ethical standards and preserve individual autonomy.
Challenges and Future Directions
Overcoming Hurdles in Clinical Translation
The translation of WJ-MSC-based therapies from the laboratory to clinical practice presents numerous challenges, including safety concerns, scalability issues, and regulatory hurdles. Addressing these challenges requires collaborative efforts among researchers, clinicians, and regulatory agencies to streamline the development and commercialization of stem cell therapies. Overcoming barriers to clinical translation is essential to realize the full therapeutic potential of WJ-MSCs and bring novel treatments to patients in need.
Harnessing Emerging Technologies
Advancements in technology offer new opportunities to enhance the efficacy and safety of WJ-MSC therapies. From gene editing tools to 3D bioprinting techniques, emerging technologies enable precise manipulation and delivery of stem cells for targeted therapeutic interventions. Integrating these technologies into existing treatment paradigms holds promise for revolutionizing regenerative medicine and addressing unmet clinical needs with unprecedented precision and efficiency.
Potential Collaborations and Interdisciplinary Approaches
Collaboration and interdisciplinary research are vital for accelerating the development and adoption of WJ-MSC-based therapies. By fostering collaboration between scientists, clinicians, engineers, and regulatory experts, synergistic partnerships can drive innovation, overcome technical challenges, and expedite the translation of research findings into clinical applications. Embracing a multidisciplinary approach holds the key to unlocking the full therapeutic potential of WJ-MSCs and ushering in a new era of regenerative medicine.
FAQs
1. How do WJ-MSCs differ from other types of stem cells?
WJ-MSCs, derived from the umbilical cord, possess unique immunomodulatory properties and higher proliferation rates compared to other types of mesenchymal stem cells. Their non-invasive extraction and low immunogenicity make them attractive candidates for therapeutic applications.
2. What are the potential risks associated with WJ-MSC therapy?
While WJ-MSC therapy holds promise for treating various medical conditions, potential risks include immune rejection, tumorigenicity, and unintended differentiation. Rigorous preclinical and clinical studies are essential to evaluate safety profiles and mitigate potential risks before widespread clinical implementation.
3. How can patients participate in clinical trials involving WJ-MSC therapy?
Patients interested in participating in clinical trials involving WJ-MSC therapy should consult with their healthcare providers and research institutions conducting trials. Participation criteria, eligibility requirements, and informed consent procedures vary depending on the specific trial protocols and regulatory guidelines.