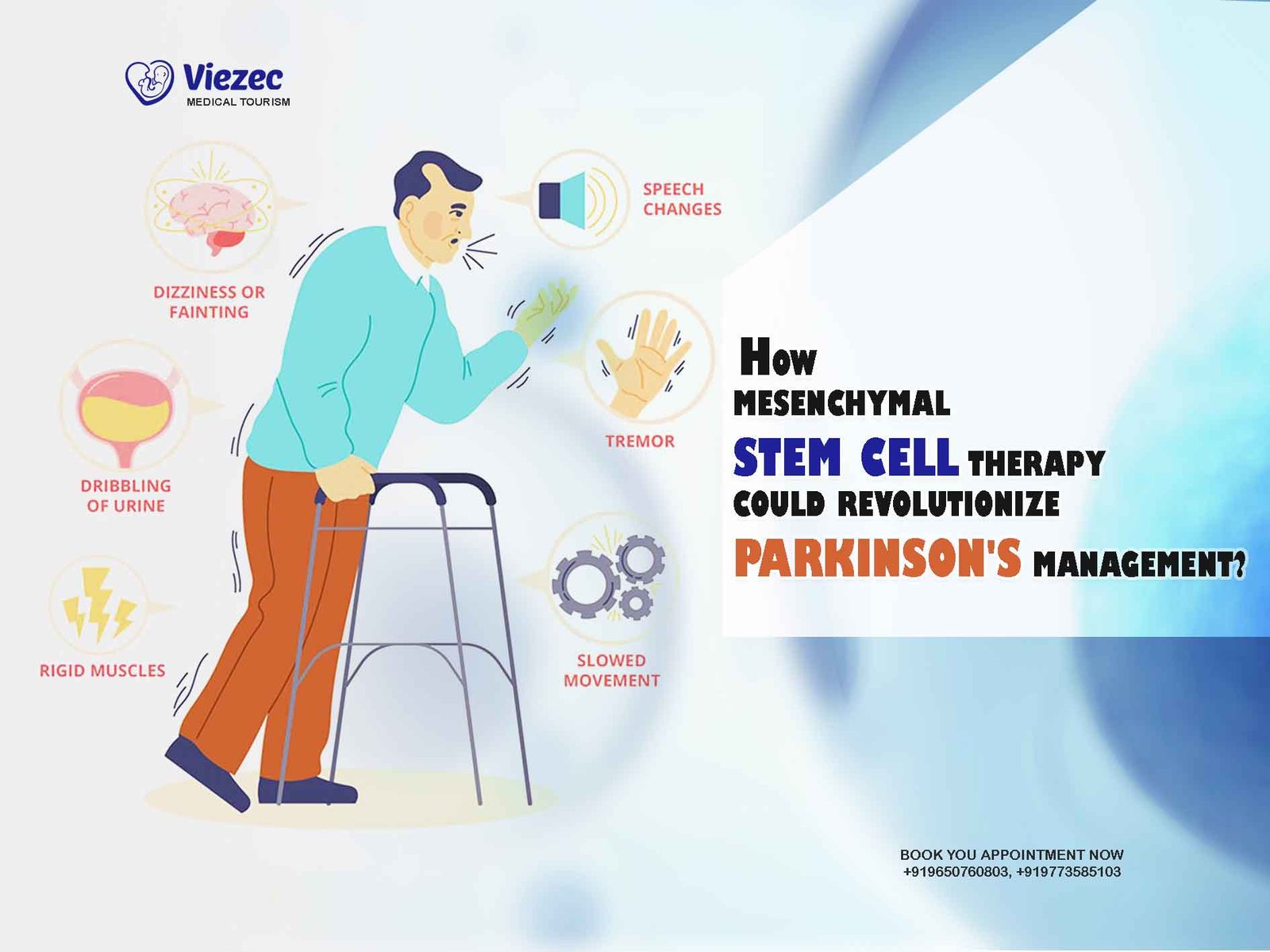
In recent years, regenerative medicine has emerged as a promising field offering potential solutions to a wide array of medical conditions. Among the various types of stem cells, human umbilical cord mesenchymal stem cells (hUC-MSCs) have garnered significant attention due to their unique properties and therapeutic potential. These cells, derived from the umbilical cord tissue, possess remarkable regenerative abilities and immunomodulatory properties, making them a focal point in the quest for novel therapeutic interventions. In this article, we delve into the intricacies of hUC-MSCs, exploring their therapeutic mechanisms and applications across different medical fields.
Understanding Human Umbilical Cord Mesenchymal Stem Cells (hUC-MSCs)
Origins and Characteristics
Human umbilical cord mesenchymal stem cells are a type of multipotent stem cell found in the Wharton’s jelly of the umbilical cord. Unlike embryonic stem cells, which are derived from the inner cell mass of blastocysts, hUC-MSCs are harvested from the umbilical cord tissue postnatally, posing fewer ethical concerns. These cells exhibit self-renewal capabilities and can differentiate into various cell types, including adipocytes, osteoblasts, chondrocytes, and neurons, among others.
Unique Properties
One of the key characteristics of hUC-MSCs is their immunomodulatory potential. These cells possess the ability to suppress immune responses through the secretion of anti-inflammatory cytokines and interaction with immune cells such as T cells, B cells, and natural killer cells. This immunomodulatory property makes them particularly attractive for the treatment of autoimmune diseases and conditions characterized by excessive inflammation.
Advantages Over Other Stem Cell Sources
Compared to other sources of mesenchymal stem cells, such as bone marrow and adipose tissue, hUC-MSCs offer several advantages. The umbilical cord tissue is readily available without invasive procedures, and the isolation of hUC-MSCs is less technically demanding compared to other sources. Moreover, hUC-MSCs exhibit higher proliferation rates and lower immunogenicity, reducing the risk of immune rejection in transplant applications.
Therapeutic Mechanisms of hUC-MSCs
Immunomodulation
One of the primary mechanisms underlying the therapeutic effects of hUC-MSCs is their immunomodulatory activity. These cells can modulate both innate and adaptive immune responses, leading to the suppression of inflammation and tissue damage. Through the secretion of factors such as transforming growth factor-beta (TGF-β), interleukin-10 (IL-10), and prostaglandin E2 (PGE2), hUC-MSCs inhibit the activation and proliferation of immune cells, thereby promoting immune tolerance and tissue repair.
Paracrine Signaling
Another important mechanism by which hUC-MSCs exert their therapeutic effects is through paracrine signaling. These cells release a wide array of bioactive molecules, including growth factors, cytokines, and chemokines, which act on neighboring cells to promote tissue regeneration and repair. By stimulating angiogenesis, reducing apoptosis, and enhancing cell proliferation, hUC-MSC-derived paracrine factors contribute to the restoration of damaged tissues in various disease conditions.
Differentiation Potential
While the paracrine effects of hUC-MSCs play a significant role in their therapeutic action, their differentiation potential also contributes to tissue regeneration. In response to specific microenvironmental cues, hUC-MSCs can differentiate into cell types relevant to the target tissue, such as neurons in neurological disorders or chondrocytes in cartilage defects. This multilineage differentiation capacity enhances the regenerative capabilities of hUC-MSCs and enables them to participate directly in tissue repair processes.
Therapeutic Applications of hUC-MSCs
Neurological Disorders
The neuroregenerative potential of hUC-MSCs has sparked interest in their application for the treatment of neurological disorders, including stroke, spinal cord injury, and neurodegenerative diseases such as Parkinson’s and Alzheimer’s. Preclinical studies have demonstrated the ability of hUC-MSCs to promote neuronal survival, enhance synaptic plasticity, and modulate neuroinflammatory responses, offering hope for novel therapeutic strategies in neurology.
Autoimmune Diseases
Autoimmune diseases arise from dysregulated immune responses targeting self-tissues, leading to chronic inflammation and tissue damage. The immunomodulatory properties of hUC-MSCs make them promising candidates for the treatment of autoimmune conditions such as rheumatoid arthritis, multiple sclerosis, and systemic lupus erythematosus. Clinical trials investigating the efficacy of hUC-MSC therapy in autoimmune diseases have shown promising results, with improvements in disease activity and reduced inflammatory markers.
Cardiovascular Disorders
Cardiovascular diseases, including myocardial infarction, heart failure, and peripheral artery disease, remain leading causes of morbidity and mortality worldwide. hUC-MSCs have emerged as potential therapeutic agents for these conditions due to their ability to promote angiogenesis, reduce myocardial fibrosis, and improve cardiac function. Clinical studies have demonstrated the safety and feasibility of hUC-MSC transplantation in patients with cardiovascular disorders, with preliminary evidence of efficacy in promoting cardiac repair and regeneration.
Orthopedic Injuries
Orthopedic injuries, such as bone fractures and cartilage defects, pose significant challenges in clinical management due to limited regenerative capacity. hUC-MSC-based therapies offer promising solutions for promoting bone and cartilage regeneration through their differentiation potential and paracrine effects. Preclinical studies have shown that hUC-MSCs can enhance fracture healing, stimulate osteogenesis, and attenuate cartilage degeneration, paving the way for the development of novel treatments for orthopedic conditions.
Challenges and Future Directions
While hUC-MSCs hold great promise as therapeutic agents, several challenges must be addressed to realize their full potential in clinical practice. Standardization of cell isolation, expansion, and characterization protocols is essential to ensure the safety and efficacy of hUC-MSC-based therapies. Additionally, optimizing delivery methods and dosing regimens, as well as elucidating the long-term safety profile of hUC-MSCs, are critical considerations for clinical translation.
Future research directions in the field of hUC-MSC therapy include the development of personalized treatment approaches based on patient-specific factors and disease characteristics. Advances in tissue engineering and biomaterials may also enhance the effectiveness of hUC-MSC-based strategies for tissue regeneration and repair. Moreover, ongoing efforts to unravel the molecular mechanisms underlying the therapeutic effects of hUC-MSCs will provide valuable insights into their mode of action and facilitate the development of targeted interventions.
Make informed Choice
Human umbilical cord mesenchymal stem cells represent a valuable resource in regenerative medicine, offering versatile therapeutic options for a wide range of medical conditions. Through their immunomodulatory properties, paracrine signaling, and differentiation potential, hUC-MSCs hold promise for the treatment of neurological disorders, autoimmune diseases, cardiovascular disorders, orthopedic injuries, and beyond. While challenges remain in harnessing the full potential of hUC-MSC-based therapies, ongoing research efforts continue to advance our understanding of these remarkable cells and their applications in clinical practice. With further innovation and collaboration, hUC-MSCs may pave the way for transformative treatments that improve patient outcomes and quality of life.