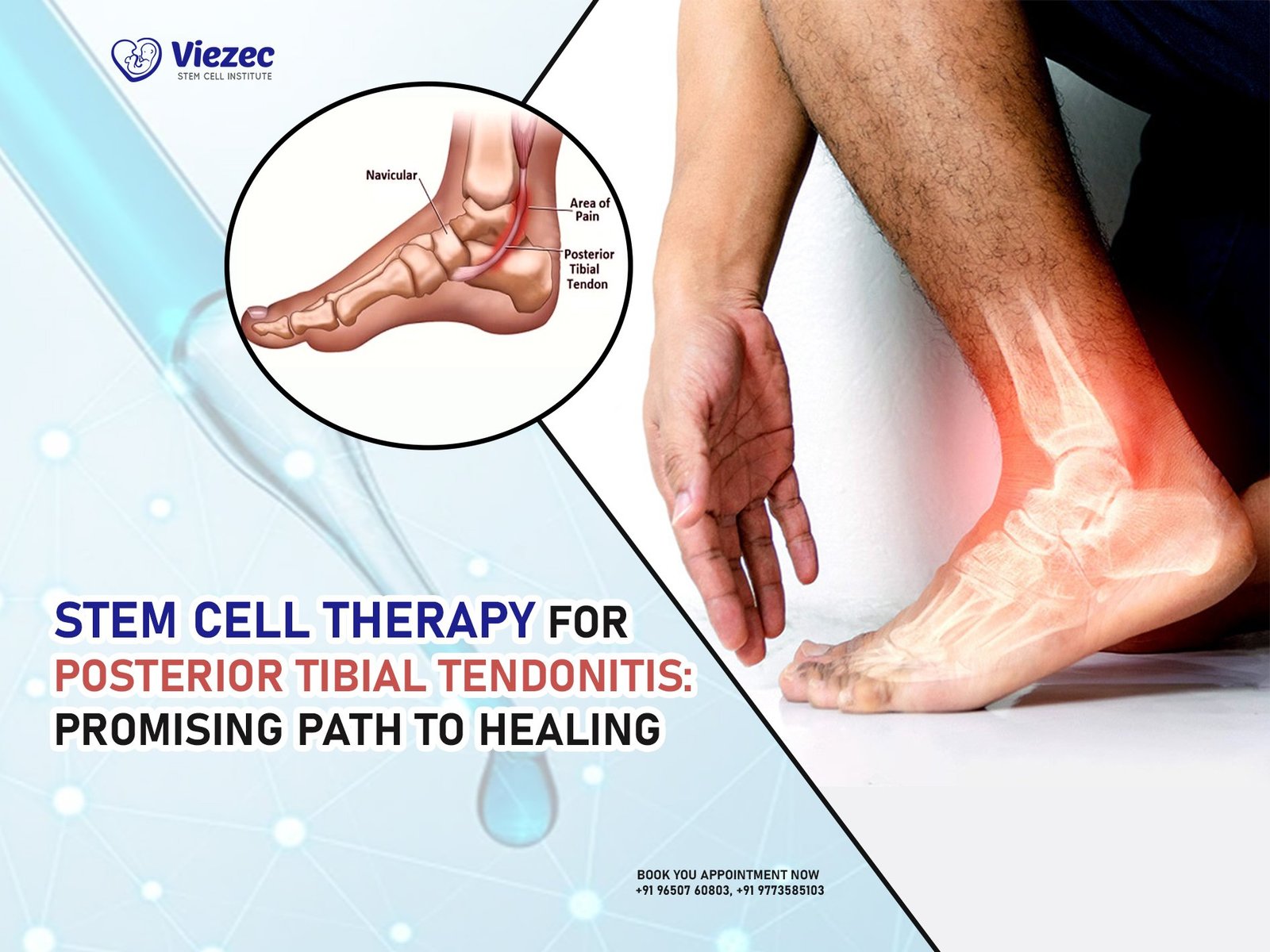
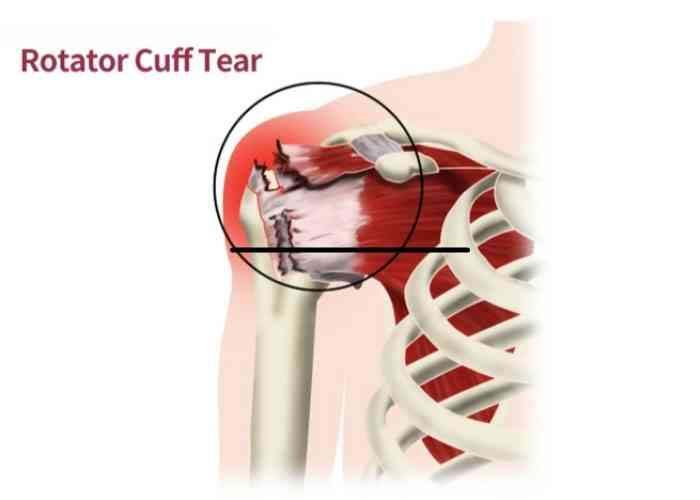
Stem cell therapy has emerged as a promising approach for treating various medical conditions, including bone fractures and defects. The concept of using stem cells to regenerate bone tissue has garnered significant attention in recent years. But what exactly is stem cell therapy, and how effective is it in bone regeneration?
Can stem cells regenerate bone?
Stem cells possess the remarkable ability to differentiate into various cell types, including bone-forming cells known as osteoblasts. When appropriately stimulated, stem cells can regenerate bone tissue, making them invaluable for repairing bone fractures and defects.
Can stem cells mend a broken bone?
Yes, stem cells have shown the potential to mend broken bones by facilitating the regeneration of new bone tissue. Through their ability to differentiate into osteoblasts and produce extracellular matrix components, stem cells play a crucial role in the bone healing process.
Does stem cell therapy really work?
Numerous preclinical and clinical studies have demonstrated the efficacy of stem cell therapy in promoting bone regeneration and fracture healing. While further research is needed to optimize protocols and address challenges, the evidence thus far suggests that stem cell therapy holds great promise for bone repair.
Is regenerative stem cell therapy safe?
Safety is a paramount concern in regenerative medicine. While stem cell therapy offers exciting potential, ensuring its safety is essential. Rigorous preclinical and clinical studies are conducted to assess the safety profile of stem cell therapies, minimizing risks associated with immunogenicity, tumorigenicity, and other potential adverse effects.
Understanding Adult Stem Cells for Bone Regeneration
Adult stem cells serve as the body’s natural reservoir for tissue regeneration and repair. Several types of adult stem cells contribute to bone regeneration, each with its unique characteristics and mechanisms.
Types of adult stem cells involved
Mesenchymal stem cells (MSCs) are the primary adult stem cells involved in bone regeneration. These multipotent cells can differentiate into various cell types, including osteoblasts, chondrocytes, and adipocytes, making them ideal candidates for bone repair.
Mechanisms of bone regeneration by adult stem cells
Adult stem cells promote bone regeneration through multiple mechanisms, including direct differentiation into bone-forming cells, secretion of growth factors and cytokines, and modulation of the immune response and inflammatory microenvironment at the injury site.
Limitations and challenges in utilizing adult stem cells
Despite their therapeutic potential, adult stem cell-based therapies face several limitations and challenges, such as limited cell availability, heterogeneity of cell populations, and variability in differentiation capacity. Overcoming these challenges is crucial for realizing the full potential of adult stem cells in bone regeneration.
Role of Mesenchymal Stem Cells (MSCs) in Bone Regeneration
Mesenchymal stem cells (MSCs) have emerged as a promising tool for bone regeneration due to their unique characteristics and differentiation potential.
Characteristics and sources of MSCs
MSCs can be isolated from various tissues, including bone marrow, adipose tissue, and umbilical cord blood. These cells exhibit self-renewal capacity and can differentiate into osteoblasts, making them valuable for bone regeneration therapies.
Differentiation potential of MSCs into bone-forming cells
Under appropriate conditions, MSCs can differentiate into osteoblasts, the primary cells responsible for bone formation. This osteogenic differentiation capacity makes MSCs an attractive option for promoting bone repair and regeneration in clinical settings.
Clinical applications and efficacy of MSC-based therapies
MSC-based therapies have shown promising results in preclinical and clinical studies for treating bone fractures, non-union defects, and other orthopedic conditions. These therapies offer a safe and effective approach to enhance bone regeneration and accelerate fracture healing.
Identifying Stem Cells for Bone Regeneration
Recent advancements in stem cell research have led to the identification of specific stem cell populations involved in bone regeneration, paving the way for personalized regenerative therapies.
Study identifies stem cell that gives rise to new bone
Recent studies have identified a subset of stem cells, known as skeletal stem cells (SSCs), responsible for generating new bone tissue. These SSCs exhibit robust osteogenic potential and play a crucial role in bone repair and regeneration processes.
Techniques and methodologies in stem cell identification
Various techniques, including flow cytometry, single-cell RNA sequencing, and lineage tracing, are employed to identify and characterize stem cell populations involved in bone regeneration. These advanced methodologies provide insights into the molecular and cellular mechanisms underlying bone repair.
Implications for personalized regenerative therapies
The identification of specific stem cell populations involved in bone regeneration has significant implications for developing personalized regenerative therapies tailored to individual patients’ needs. By understanding the unique properties of different stem cell populations, researchers can optimize treatment strategies for enhanced bone repair outcomes.
Mechanisms of Stem Cell-Mediated Bone Repair
Stem cell-mediated bone repair involves complex cellular processes and molecular interactions that orchestrate the regeneration of damaged bone tissue.
Cellular processes involved in bone repair by stem cells
Stem cells contribute to bone repair through various cellular processes, including proliferation, differentiation, migration, and extracellular matrix deposition. These processes are tightly regulated to ensure proper bone healing and regeneration.
Signaling pathways and molecular interactions in bone regeneration
Multiple signaling pathways, such as Wnt, BMP, and Notch signaling, play critical roles in regulating stem cell behavior and bone regeneration. Crosstalk between different signaling pathways coordinates the activities of various cell types involved in the bone repair process.
Factors influencing the efficiency of stem cell-based repair
Several factors influence the efficiency of stem cell-based bone repair, including the local microenvironment, patient age and health status, and the delivery method and timing of stem cell therapy. Understanding these factors is essential for optimizing treatment protocols and improving clinical outcomes.
Enhancing Stem Cell Therapy for Bone Fractures
To maximize the therapeutic potential of stem cell therapy for bone fractures, researchers are exploring various strategies to enhance stem cell delivery and improve bone regeneration outcomes.
Optimization strategies for stem cell delivery
Optimizing stem cell delivery methods, such as scaffold-based approaches, direct injection, and tissue engineering techniques, can improve cell retention, viability, and integration at the injury site. These optimization strategies enhance the effectiveness of stem cell therapy for bone fracture repair.
Biomaterial scaffolds and tissue engineering approaches
Biomaterial scaffolds provide a supportive framework for stem cell growth and differentiation, mimicking the native extracellular matrix of bone tissue. Tissue engineering approaches combine stem cells with biomaterial scaffolds to create functional tissue constructs for repairing bone defects and fractures.
Combinatorial therapies to augment bone fracture healing
Combining stem cell therapy with other therapeutic modalities, such as growth factors, cytokines, and gene therapy, can synergistically enhance bone fracture healing. These combinatorial approaches target multiple aspects of the bone repair process to accelerate healing and improve functional outcomes.
Clinical Applications and Trials in Stem Cell-Based Bone Regeneration
Current status of clinical trials utilizing stem cells for bone repair
Clinical trials exploring the efficacy of stem cell therapy in bone regeneration have gained momentum in recent years. Various types of stem cells, including mesenchymal stem cells (MSCs) derived from bone marrow, adipose tissue, and umbilical cord, have been investigated for their potential in promoting bone healing. These trials encompass a wide range of orthopedic conditions, such as non-union fractures, osteonecrosis, and osteoarthritis, demonstrating the versatility of stem cell-based approaches in addressing different pathologies.
Successes, challenges, and future directions
While some clinical trials have shown promising results in enhancing bone regeneration and improving patient outcomes, challenges remain in translating these findings into widespread clinical practice. Factors such as optimal cell source selection, dosing regimens, and delivery methods need to be standardized to ensure reproducibility and efficacy across diverse patient populations. Moreover, the long-term safety and durability of stem cell-based interventions require further investigation to address concerns regarding potential adverse effects and treatment sustainability.
Regulatory considerations and ethical implications
The regulatory landscape surrounding stem cell therapy for bone regeneration is complex, with stringent requirements for clinical translation and commercialization. Regulatory agencies worldwide closely monitor the development and implementation of stem cell-based interventions to safeguard patient safety and ensure ethical conduct. Ethical considerations, such as informed consent, patient confidentiality, and equitable access to treatment, also play a crucial role in shaping the ethical framework for stem cell research and clinical practice.
Assessment of Stem Cell Therapy Efficacy in Bone Regeneration
Preclinical models for evaluating stem cell therapy outcomes
Preclinical studies utilizing animal models provide valuable insights into the efficacy and mechanism of action of stem cell therapy in bone regeneration. These models allow researchers to assess various parameters, including bone formation, remodeling, and integration with host tissue, to determine the therapeutic potential of different stem cell sources and delivery strategies. By replicating human pathologies in controlled experimental settings, preclinical models bridge the gap between benchtop research and clinical translation, facilitating the development of novel treatment modalities.
Imaging techniques for monitoring bone healing and regeneration
Advanced imaging modalities, such as computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET), offer non-invasive tools for evaluating bone healing and regeneration in real-time. These imaging techniques enable clinicians to visualize changes in bone morphology, density, and vascularity following stem cell therapy, providing valuable information for treatment planning and monitoring. By accurately assessing the progress of bone repair over time, imaging plays a crucial role in optimizing therapeutic outcomes and identifying potential complications early in the treatment process.
Long-term follow-up studies and patient-reported outcomes
Long-term follow-up studies are essential for assessing the durability and sustainability of stem cell-based interventions in bone regeneration. These studies track patient outcomes, including functional recovery, pain relief, and quality of life, over an extended period to evaluate the long-term efficacy and safety of treatment. Patient-reported outcomes, such as satisfaction with treatment outcomes and adherence to rehabilitation protocols, provide valuable insights into the subjective experiences and preferences of individuals undergoing stem cell therapy. By incorporating patient perspectives into clinical research, long-term follow-up studies enhance the relevance and applicability of stem cell-based interventions in real-world healthcare settings.
Safety Considerations in Regenerative Stem Cell Therapy
Risk assessment and management strategies
Safety remains a paramount concern in the development and clinical implementation of stem cell therapy for bone regeneration. Risk assessment strategies, including comprehensive preclinical testing, rigorous quality control measures, and standardized protocols for cell processing and delivery, are essential for minimizing potential adverse effects and ensuring patient safety. Close monitoring of patients throughout the treatment process, coupled with prompt intervention in case of complications, is crucial for mitigating risks and optimizing treatment outcomes.
Immunogenicity and tumorigenicity concerns
Immunogenic and tumorigenic potential pose significant challenges to the safety and efficacy of stem cell-based interventions in bone regeneration. Host immune responses to transplanted cells, as well as the risk of aberrant cell proliferation and malignant transformation, necessitate thorough preclinical evaluation and vigilant post-treatment monitoring. Strategies for mitigating immunogenicity and tumorigenicity, such as immunomodulatory cell priming and genetic modification techniques, hold promise for enhancing the safety profile of stem cell therapy and minimizing the risk of adverse events.
Regulatory frameworks and guidelines for ensuring patient safety
Regulatory frameworks and guidelines govern the ethical conduct and safety standards of stem cell therapy for bone regeneration. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), enforce stringent regulations for the development, manufacturing, and clinical use of stem cell-based products. Compliance with Good Manufacturing Practice (GMP) standards, adherence to ethical guidelines for human subjects research, and transparent reporting of clinical trial data are essential for ensuring patient safety and regulatory compliance. By upholding rigorous regulatory standards, stakeholders can instill confidence in the safety and efficacy of stem cell-based interventions and accelerate their translation into clinical practice.
Future Perspectives and Emerging Technologies
Novel approaches in stem cell isolation and manipulation
Advances in stem cell isolation and manipulation techniques hold promise for enhancing the therapeutic potential and scalability of stem cell-based interventions for bone regeneration. Innovative methods for sourcing and expanding stem cells, such as induced pluripotent stem cells (iPSCs) and cell reprogramming technologies, offer alternative strategies for overcoming limitations associated with traditional cell sources. Moreover, advancements in gene editing and tissue engineering enable precise control over stem cell behavior and differentiation, paving the way for personalized regenerative therapies tailored to individual patient needs.
Advancements in biomaterial design for enhanced bone regeneration
Biomaterials play a pivotal role in supporting stem cell survival, proliferation, and differentiation within the bone microenvironment. Emerging biomaterials, such as biocompatible scaffolds, growth factor-releasing matrices, and 3D-printed constructs, offer versatile platforms for delivering stem cells to the site of injury and promoting tissue regeneration. By mimicking the native extracellular matrix of bone tissue and providing structural support for cell attachment and growth, next-generation biomaterials enhance the therapeutic efficacy and integration of stem cell-based interventions, leading to improved outcomes for patients with orthopedic conditions.
Integration of stem cell therapy with other regenerative medicine modalities
The integration of stem cell therapy with complementary regenerative medicine modalities holds immense potential for synergistic tissue repair and regeneration. Combined approaches, such as cell-based therapies, growth factor supplementation, and tissue engineering strategies, capitalize on the unique properties of different regenerative agents to enhance bone healing and functional recovery. By harnessing the collective power of stem cells, biomaterials, and bioactive factors, multidisciplinary regenerative strategies offer holistic solutions for addressing complex orthopedic challenges and improving patient outcomes across diverse clinical settings.