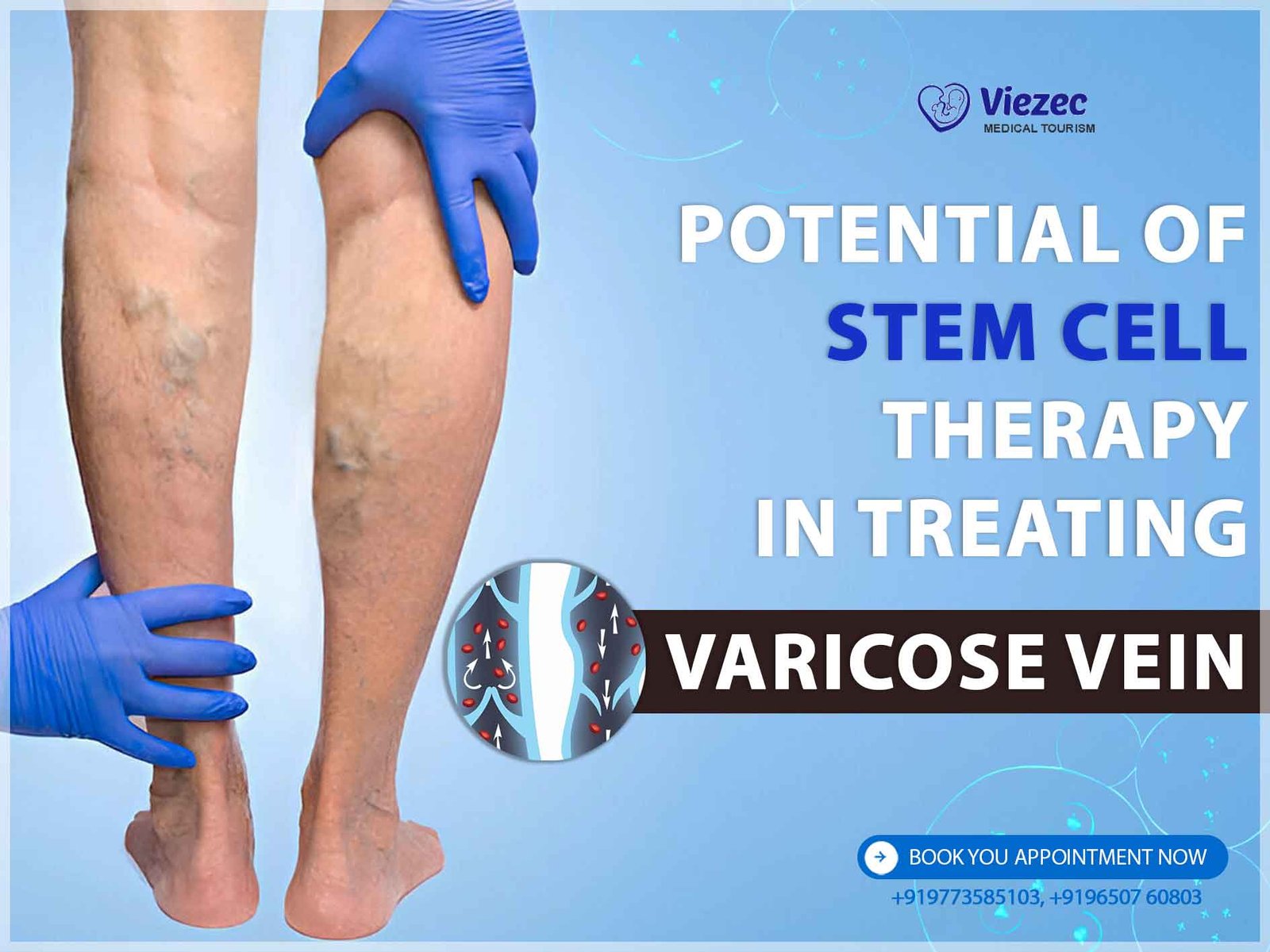
Varicose veins, a common vascular disorder, occur when veins become enlarged, swollen, and twisted, often appearing blue or dark purple. This condition primarily affects the legs and feet due to increased pressure on the veins, weakening their walls and valves. Symptoms include pain, swelling, and discomfort, impacting quality of life.
Limitations of Current Treatments
Conventional treatments for varicose veins include compression stockings, lifestyle modifications, and surgical procedures like vein stripping and laser therapy. However, these approaches may not address underlying pathophysiological mechanisms, leading to recurrence and incomplete symptom relief.
Emergence of Stem Cell Therapy
Stem cell therapy presents a promising alternative by harnessing the regenerative potential of stem cells to repair damaged blood vessels and improve venous function. This innovative approach aims to address the limitations of traditional treatments and provide long-term relief for patients with varicose veins.
Anatomy and Pathophysiology of Varicose Veins
Venous System Overview
The venous system comprises a network of blood vessels responsible for returning deoxygenated blood from the body’s tissues back to the heart. Varicose veins develop when valves within these veins fail to function properly, allowing blood to pool and veins to dilate, leading to the characteristic bulging and twisting seen in varicose veins.
Pathophysiological Changes in Varicose Veins
Chronic venous insufficiency, the underlying pathology of varicose veins, involves venous hypertension, inflammation, and structural changes in vein walls. These alterations contribute to venous valve dysfunction and impaired blood flow, exacerbating symptoms and increasing the risk of complications such as venous ulcers and thrombosis.
Role of Stem Cells in Tissue Regeneration
Stem cells, with their ability to differentiate into various cell types and promote tissue repair, offer a potential solution for restoring normal venous function in patients with varicose veins. By targeting the underlying pathophysiology, stem cell therapy aims to reverse vascular damage and improve venous circulation, alleviating symptoms and preventing disease progression.
Stem Cell Biology and Types Relevant to Varicose Veins
Overview of Stem Cells
Stem cells are undifferentiated cells capable of self-renewal and differentiation into specialized cell types. They exist in various tissues throughout the body and play essential roles in development, homeostasis, and tissue repair. Understanding the different types of stem cells is crucial for harnessing their therapeutic potential in treating varicose veins.
Mesenchymal Stem Cells (MSCs)
MSCs, found in bone marrow, adipose tissue, and other sources, exhibit multipotent differentiation capabilities and secrete factors that promote angiogenesis, immunomodulation, and tissue regeneration. These properties make MSCs attractive candidates for treating vascular diseases, including varicose veins.
Endothelial Progenitor Cells (EPCs)
EPCs are a subset of stem cells with endothelial lineage commitment, capable of differentiating into mature endothelial cells and contributing to vascular repair and neovascularization. Harnessing the regenerative potential of EPCs holds promise for enhancing vascular function and improving outcomes in patients with varicose veins.
Induced Pluripotent Stem Cells (iPSCs)
iPSCs are reprogrammed adult cells that exhibit pluripotency, allowing them to differentiate into any cell type in the body. While still in the early stages of research, iPSCs offer a renewable source of patient-specific stem cells for personalized regenerative therapies in varicose vein treatment.
Take the First Step Towards Healing
Mechanisms of Action of Stem Cells in Varicose Vein Treatment
Angiogenesis and Neovascularization
Stem cells promote angiogenesis, the formation of new blood vessels, by secreting growth factors and cytokines that stimulate endothelial cell proliferation and migration. This process restores blood flow to ischemic tissues, facilitating healing and reducing symptoms associated with varicose veins.
Anti-inflammatory Effects
Stem cells exert anti-inflammatory effects by modulating immune responses and suppressing pro-inflammatory cytokines. By attenuating chronic inflammation in venous tissues, stem cell therapy mitigates tissue damage and promotes a favorable microenvironment for vascular repair and remodeling.
Extracellular Matrix Remodeling
Stem cells contribute to extracellular matrix remodeling by secreting matrix metalloproteinases and other enzymes that degrade aberrant matrix components and promote tissue restructuring. This remodeling process enhances venous wall integrity and improves venous function in patients with varicose veins.
Immunomodulation
Stem cells regulate immune cell activity and promote tissue tolerance through paracrine signaling and cell-to-cell interactions. By modulating immune responses, stem cell therapy reduces inflammation, minimizes tissue damage, and enhances the efficacy of vascular repair mechanisms in varicose vein treatment.
Preclinical Studies and Animal Models
Efficacy in Animal Models
Preclinical studies have demonstrated the therapeutic efficacy of stem cell therapy in animal models of venous insufficiency and varicose veins. These studies have shown improvements in venous function, reduced vein diameter, and increased neovascularization following stem cell administration, supporting its potential for clinical translation.
Safety Profile
Safety assessments in preclinical models have revealed favorable safety profiles for stem cell therapy, with minimal adverse effects reported. Long-term follow-up studies have confirmed the absence of tumorigenicity or ectopic tissue formation, further supporting the safety and feasibility of stem cell-based interventions for varicose veins.
Optimal Cell Type and Delivery Methods
Optimizing the selection of stem cell types and delivery methods is essential for maximizing therapeutic outcomes in varicose vein treatment. Comparative studies evaluating different cell sources, dosage regimens, and delivery routes are needed to identify the most effective strategies for clinical application.
Clinical Trials and Evidence-Based Findings
Phase I Trials: Safety and Feasibility
Early-phase clinical trials have demonstrated the safety and feasibility of stem cell therapy in patients with varicose veins. These trials have focused on establishing optimal dosing, safety endpoints, and delivery techniques, laying the foundation for subsequent efficacy studies.
Phase II Trials: Efficacy and Dosage Optimization
Phase II trials have investigated the efficacy of stem cell therapy in improving venous function, reducing symptoms, and preventing disease progression in patients with varicose veins. These trials have provided valuable insights into optimal dosage regimens and treatment protocols for further evaluation in larger-scale studies.
Phase III Trials: Large-Scale Efficacy Studies
Large-scale phase III trials are underway to evaluate the long-term efficacy and safety of stem cell therapy in diverse patient populations with varicose veins. These pivotal studies aim to provide robust evidence supporting the clinical benefits of stem cell-based interventions and guide regulatory approval and clinical practice.
Meta-Analyses and Systematic Reviews
Meta-analyses and systematic reviews of clinical data are essential for synthesizing evidence, evaluating treatment outcomes, and identifying factors influencing therapeutic efficacy in varicose vein management. These analyses inform clinical decision-making and guideline development, shaping the future direction of stem cell therapy in vascular medicine.
Ready to Learn More About Your Treatment Options?
Stem Cell Sources and Harvesting Techniques
Bone Marrow Aspirate
Bone marrow aspiration is a common method for obtaining mesenchymal stem cells, which reside within the bone marrow stroma. This minimally invasive procedure involves extracting bone marrow from the iliac crest or other sites, followed by isolation and expansion of MSCs for therapeutic use in varicose vein treatment.
Adipose Tissue Harvesting
Adipose tissue harvesting involves liposuction or surgical excision of subcutaneous fat deposits to obtain adipose-derived stem cells. Adipose tissue is rich in MSCs and other progenitor cells, making it a valuable source for regenerative therapies targeting vascular disorders such as varicose veins.
Cord Blood Collection
Cord blood collection at birth offers a non-invasive method for obtaining hematopoietic stem cells and endothelial progenitor cells from the umbilical cord. Cord blood banking provides a readily available source of stem cells for allogeneic transplantation and regenerative medicine applications, including varicose vein therapy.
Induced Pluripotent Stem Cell Generation
Induced pluripotent stem cells are generated by reprogramming somatic cells using defined transcription factors, such as Oct4, Sox2, Klf4, and c-Myc. This technology enables the generation of patient-specific stem cell lines for personalized therapies and disease modeling in varicose vein research.
Delivery Strategies for Stem Cell Therapy
Direct Injection into Varicose Veins
Direct injection of stem cells into varicose veins enables targeted delivery to sites of vascular pathology, facilitating localized tissue repair and regeneration. This minimally invasive approach offers precise control over cell dosage and distribution, enhancing therapeutic efficacy and reducing systemic side effects.
Intravenous Infusion
Intravenous infusion of stem cells involves systemic delivery via peripheral venous access, allowing widespread distribution throughout the circulatory system. While less invasive than direct injection, intravenous delivery may result in lower cell retention at target sites and require higher cell doses to achieve therapeutic effects in varicose vein treatment.
Scaffold-Based Delivery Systems
Scaffold-based delivery systems provide a supportive matrix for stem cell attachment, proliferation, and differentiation, promoting tissue integration and functional restoration. These scaffolds can be implanted directly into venous defects or used as carriers for controlled release of stem cells and bioactive factors in varicose vein repair.
Catheter-Based Delivery Techniques
Catheter-based delivery techniques enable precise placement of stem cells using minimally invasive procedures, such as balloon catheterization and endovascular stenting. These approaches allow targeted delivery to specific venous segments and facilitate cell engraftment and integration into vascular tissues, enhancing therapeutic outcomes for varicose veins.
Long-Term Safety and Efficacy Monitoring
Long-term safety and efficacy monitoring are essential for evaluating the durability of therapeutic effects and identifying potential adverse events associated with stem cell therapy in varicose vein management. Comprehensive follow-up studies and post-marketing surveillance initiatives are needed to ensure patient safety and optimize treatment protocols.
Standardization of Protocols
Standardization of stem cell isolation, expansion, and delivery protocols is critical for ensuring consistency and reproducibility across clinical trials and treatment centers. Collaborative efforts among researchers, clinicians, and regulatory agencies are necessary to establish consensus guidelines and best practices for stem cell-based interventions in varicose vein therapy.
Combination Therapies and Personalized Medicine
Exploring combination therapies and personalized medicine approaches holds promise for enhancing the efficacy of stem cell therapy in varicose vein treatment. Combinatorial strategies involving stem cells, growth factors, biomaterials, and pharmacological agents may synergistically promote vascular regeneration and improve patient outcomes in personalized treatment regimens.
Start Your Stem Cell Therapy Journey Now
Regulatory Landscape and Commercialization
FDA and EMA Regulations
Regulatory agencies such as the FDA and EMA oversee the approval and marketing of stem cell-based therapies for varicose veins and other medical indications. Rigorous preclinical and clinical evaluation is required to demonstrate safety, efficacy, and quality control compliance before regulatory approval and commercialization.
Intellectual Property Rights
Intellectual property rights play a significant role in incentivizing innovation and investment in stem cell research and development. Patents, trademarks, and licensing agreements protect novel technologies and therapeutic approaches, enabling companies to commercialize stem cell products and secure market exclusivity in varicose vein therapy.
Market Analysis and Commercial Potential
Market analysis indicates growing demand for innovative treatments for vascular disorders, including varicose veins, driven by aging populations and rising healthcare expenditures worldwide. Stem cell therapy offers a lucrative market opportunity for biotechnology and pharmaceutical companies seeking to capitalize on emerging regenerative medicine technologies.
Industry Partnerships and Collaborations
Industry partnerships and collaborations facilitate the translation of stem cell research into clinical applications and commercial products for varicose vein treatment. Collaborative initiatives between academia, government agencies, and private sector stakeholders accelerate the development and commercialization of novel therapies, benefiting patients and healthcare providers.
Conclusion and Perspectives
Directions and Research Priorities
Future research directions in stem cell therapy for varicose veins include optimizing cell sources, delivery methods, and treatment protocols to maximize therapeutic outcomes and minimize adverse events. Long-term studies are needed to evaluate the durability and sustainability of treatment effects and refine patient selection criteria for personalized medicine approaches.
Patient Education and Awareness Initiatives
Patient education and awareness initiatives play a crucial role in fostering informed decision-making and promoting acceptance of stem cell therapy in varicose vein management. Healthcare providers should engage patients in discussions about treatment options, benefits, risks, and realistic expectations to empower them in making well-informed choices about their care.
Ethical Considerations and Social Implications
Ethical considerations and social implications surrounding stem cell therapy in varicose vein treatment encompass issues related to patient consent, privacy, equity, and access to healthcare services. Transparent communication, ethical governance, and equitable distribution of resources are essential for ensuring responsible innovation and equitable healthcare delivery in regenerative medicine.
Stem cell therapy represents a paradigm shift in the management of varicose veins, offering a transformative approach to vascular regeneration and symptom relief. With ongoing advancements in stem cell research, clinical translation, and regulatory oversight, the future holds immense promise for harnessing the full potential of stem cells in improving venous health and enhancing quality of life for patients worldwide.