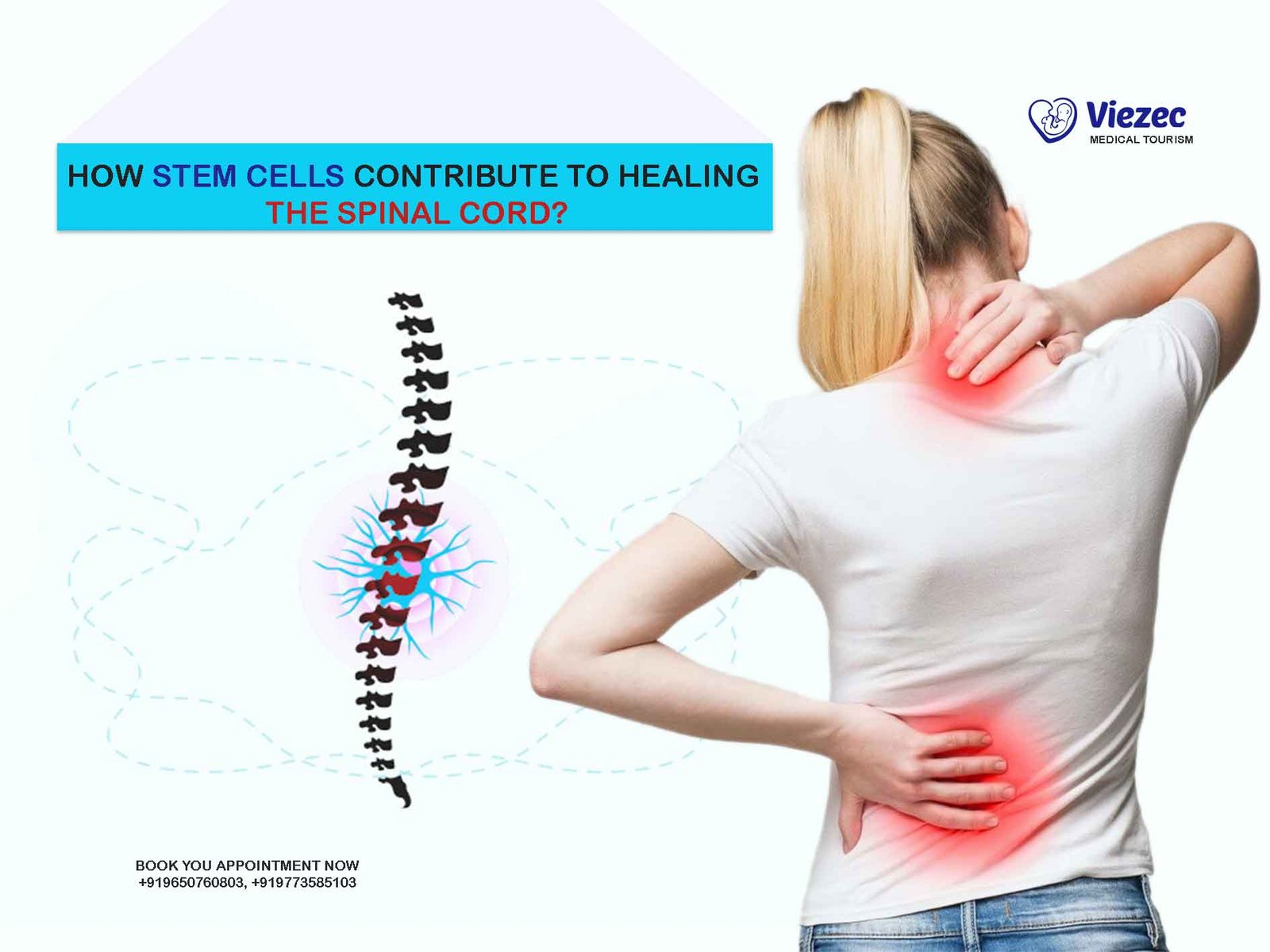
Spinal cord injury (SCI) is a devastating condition characterized by damage to the spinal cord resulting in partial or complete loss of sensory, motor, and autonomic function below the level of injury. It often leads to profound disabilities and challenges in daily life. The causes of SCI vary, ranging from traumatic incidents such as accidents or falls to non-traumatic conditions like tumors or infections. Regardless of the cause, the impact on individuals’ lives can be profound, necessitating innovative approaches for treatment and rehabilitation.
Understanding the Complexity of Spinal Cord Damage
The spinal cord, a crucial component of the central nervous system, is responsible for transmitting signals between the brain and the rest of the body. When the spinal cord is injured, disruptions occur in these signal pathways, leading to functional impairments. The complexity of spinal cord damage extends beyond the initial injury, as secondary mechanisms, including inflammation, oxidative stress, and scar formation, further exacerbate tissue damage and hinder recovery. Consequently, developing effective therapies necessitates a comprehensive understanding of the intricate cellular and molecular processes involved in SCI pathophysiology.
Current Treatment Limitations and the Need for Innovation
Despite advances in medical care, treatment options for SCI remain limited. Traditional approaches focus primarily on symptom management and rehabilitation to improve quality of life for affected individuals. However, these methods often fall short in promoting substantial neurological recovery. The lack of effective interventions underscores the urgent need for innovative treatment modalities capable of addressing the underlying mechanisms of spinal cord damage and facilitating tissue repair.
Overview of Stem Cell Therapy as a Promising Approach
Stem cell therapy has emerged as a promising strategy for treating SCI by harnessing the regenerative potential of stem cells to promote tissue repair and functional recovery. Stem cells possess unique properties, including the ability to self-renew and differentiate into various cell types, making them ideal candidates for regenerative medicine applications. In the context of SCI, stem cell therapy offers the potential to replace damaged cells, modulate inflammatory responses, and promote neural regeneration, thereby restoring neurological function.
Fundamentals of Stem Cells
Defining Stem Cells: Types and Properties
Stem cells are undifferentiated cells capable of proliferating and differentiating into specialized cell types. They can be broadly categorized into embryonic stem cells (ESCs), adult stem cells, and induced pluripotent stem cells (iPSCs). ESCs are derived from the inner cell mass of early-stage embryos and possess the broadest differentiation potential. Adult stem cells, found in various tissues throughout the body, contribute to tissue homeostasis and repair. iPSCs are generated by reprogramming somatic cells to an embryonic-like state, offering the versatility of ESCs without ethical concerns associated with embryonic sources.
Sources of Stem Cells for Spinal Cord Regeneration
Stem cells for SCI therapy can be obtained from various sources, including embryonic tissues, adult tissues, and reprogrammed somatic cells. Embryonic stem cells are typically derived from surplus embryos generated during in vitro fertilization procedures. Adult stem cells, such as mesenchymal stem cells (MSCs) or neural stem cells (NSCs), can be isolated from tissues like bone marrow, adipose tissue, or the central nervous system. iPSCs are generated by introducing specific transcription factors into somatic cells, reprogramming them into a pluripotent state suitable for differentiation into neural lineages.
Mechanisms of Stem Cell Differentiation and Integration
Upon transplantation into the injured spinal cord, stem cells undergo differentiation into neural progenitors or mature neural cells, such as neurons, astrocytes, or oligodendrocytes. The local microenvironment within the injured spinal cord plays a crucial role in guiding stem cell fate determination and promoting functional integration into existing neural circuits. Additionally, stem cells secrete various trophic factors and cytokines that exert neuroprotective and immunomodulatory effects, fostering an environment conducive to tissue repair and regeneration.
Stem Cell Therapy for Spinal Cord Repair
Types of Stem Cell Therapies: Embryonic, Adult, and Induced Pluripotent Stem Cells
Several types of stem cells have been investigated for their therapeutic potential in SCI. ESCs offer the advantage of pluripotency but are associated with ethical concerns and immune rejection. Adult stem cells, including MSCs and NSCs, are more readily available and exhibit immunomodulatory properties, making them attractive candidates for transplantation. iPSCs combine the advantages of ESCs with patient-specific compatibility, mitigating concerns regarding immune rejection and ethical considerations.
Delivery Methods: Intrathecal Injection, Intravenous Infusion, and Surgical Implantation
Stem cells can be delivered into the injured spinal cord using various techniques, including intrathecal injection, intravenous infusion, or surgical implantation. Intrathecal injection involves the direct administration of stem cells into the cerebrospinal fluid surrounding the spinal cord, allowing for widespread distribution within the central nervous system. Intravenous infusion delivers stem cells systemically, enabling access to the injured site via circulation. Surgical implantation entails the direct transplantation of stem cells into the spinal cord tissue, facilitating precise localization and integration within the lesion site.
Challenges and Considerations in Stem Cell Transplantation
Despite the therapeutic potential of stem cell therapy, several challenges must be addressed to optimize its efficacy and safety in clinical applications. These include issues related to cell survival, differentiation, and integration within the host tissue. Additionally, concerns regarding tumorigenicity, immune rejection, and ethical considerations necessitate careful consideration in the design and implementation of stem cell-based interventions for SCI.
Mechanisms of Action in Spinal Cord Healing
Neuroprotection: Stem Cells as Trophic Factor Factories
Stem cells secrete a variety of trophic factors, including neurotrophins, cytokines, and growth factors, which exert neuroprotective effects on injured neurons and promote cell survival. These trophic factors modulate inflammatory responses, reduce oxidative stress, and promote angiogenesis, creating a favorable microenvironment for tissue repair and regeneration within the injured spinal cord.
Neuronal Replacement and Axonal Regeneration
One of the primary goals of stem cell therapy in SCI is to promote neuronal replacement and axonal regeneration, thereby restoring functional connectivity within the damaged neural circuits. Transplanted stem cells differentiate into neural progenitors or mature neurons, integrating into the existing neural network and forming synapses with host neurons. Additionally, stem cells secrete factors that stimulate axonal outgrowth and guidance, facilitating the formation of new connections across the injury site.
Modulation of Inflammatory Responses and Scar Formation
SCI triggers a cascade of inflammatory events, leading to secondary tissue damage and scar formation, which impedes axonal regeneration and tissue repair. Stem cells exert immunomodulatory effects by suppressing inflammatory cytokine production, promoting anti-inflammatory signaling pathways, and modulating immune cell function. Furthermore, stem cells secrete factors that facilitate the breakdown of scar tissue and promote tissue remodeling, creating a permissive environment for axonal regeneration and functional recovery.
Preclinical and Clinical Evidence
Promising Results from Animal Studies: Regaining Motor Function and Sensory Perception
Preclinical studies in animal models of SCI have demonstrated the efficacy of stem cell therapy in promoting tissue repair and functional recovery. Transplanted stem cells have been shown to differentiate into neural cell types, integrate into host tissue, and promote axonal regeneration across the injury site. Furthermore, functional improvements in motor function, sensory perception, and bladder control have been observed following stem cell transplantation, highlighting the therapeutic potential of this approach in SCI.
Translational Challenges: Efficacy and Safety Considerations
Despite promising results in preclinical studies, translating stem cell therapy from bench to bedside presents numerous challenges. Ensuring the efficacy, safety, and long-term viability of stem cell-based interventions in human patients remains a critical priority. Addressing concerns related to cell survival, tumorigenicity, immune rejection, and ethical considerations is essential for advancing stem cell therapy towards clinical translation and widespread adoption.
Overview of Clinical Trials
Assessing the Feasibility and Long-Term Outcomes
Clinical trials evaluating the safety and efficacy of stem cell therapy in SCI are underway worldwide, aiming to provide insights into its therapeutic potential and long-term outcomes in human patients. These trials encompass various stem cell sources, delivery methods, and patient populations, with a focus on assessing functional improvements, neurological recovery, and quality of life following treatment. Additionally, ongoing research endeavors seek to elucidate the mechanisms underlying stem cell-mediated tissue repair and identify biomarkers predictive of treatment response.
Bioengineering Approaches Enhancing Stem Cell Therapy
Biomaterial Scaffolds: Providing Structural Support and Guidance
Bioengineering strategies, such as biomaterial scaffolds, offer innovative solutions to enhance the efficacy of stem cell therapy in SCI. Scaffolds provide structural support and guidance to transplanted stem cells, facilitating their localization, survival, and integration within the injured spinal cord. Furthermore, scaffolds can be engineered to mimic the native extracellular matrix, promoting cell adhesion, migration, and differentiation, thereby enhancing tissue regeneration and functional recovery.
Gene Therapy: Augmenting Stem Cell Functions and Survival
Gene therapy holds promise as a complementary approach to enhance the therapeutic potential of stem cell therapy in SCI. By genetically modifying stem cells to overexpress neurotrophic factors or anti-inflammatory cytokines, gene therapy can augment their trophic factor secretion and immunomodulatory properties, enhancing their neuroprotective effects and promoting tissue repair. Additionally, gene editing technologies offer the possibility of correcting genetic mutations associated with inherited neurological disorders, further expanding the scope of stem cell-based interventions in regenerative medicine.
Combination Therapies: Synergizing Stem Cells with Physical Rehabilitation and Pharmacological Interventions
Combination therapies that synergize stem cell transplantation with other treatment modalities, such as physical rehabilitation and pharmacological interventions, offer a multifaceted approach to SCI management. Physical rehabilitation programs aim to optimize functional recovery by promoting neuroplasticity, strengthening weakened muscles, and improving mobility and coordination. Pharmacological agents, including growth factors, anti-inflammatory drugs, and immunomodulators, complement stem cell therapy by targeting specific pathways involved in tissue repair and regeneration, thereby enhancing overall treatment outcomes.
Ethical and Regulatory Considerations
Ethical Implications of Stem Cell Research and Therapy
The ethical considerations surrounding stem cell research and therapy are multifaceted and encompass various issues, including embryo destruction, informed consent, and patient autonomy. The use of embryonic stem cells raises ethical concerns regarding the destruction of human embryos and the potential for exploitation of vulnerable populations. Furthermore, the commercialization of stem cell therapies raises questions regarding accessibility, equity, and affordability, highlighting the need for transparent regulatory frameworks and ethical guidelines to ensure responsible conduct and equitable distribution of innovative treatments.
Regulatory Frameworks: Ensuring Safety and Efficacy in Clinical Applications
Regulatory agencies play a crucial role in overseeing the development and implementation of stem cell-based therapies, ensuring their safety, efficacy, and ethical conduct in clinical settings. Regulatory frameworks vary across jurisdictions, with stringent requirements for preclinical testing, clinical trial approval, and post-market surveillance to safeguard patient welfare and public health. Additionally, regulatory agencies collaborate with researchers, clinicians, and industry stakeholders to streamline the translation of stem cell therapies from bench to bedside, balancing the imperative for innovation with the imperative for safety and ethical integrity.
Patient Perspectives and Informed Consent in Stem Cell Trials
Informed consent is a cornerstone of ethical stem cell research and clinical practice, empowering patients to make informed decisions about their participation in clinical trials and treatment options. Providing comprehensive information about the risks, benefits, and alternatives to stem cell therapy is essential for ensuring patient autonomy and minimizing therapeutic misconceptions. Furthermore, fostering open communication and dialogue between patients, healthcare providers, and researchers promotes trust, transparency, and shared decision-making, enhancing the ethical conduct and integrity of stem cell-based interventions in SCI.
Future Directions and Challenges
Advancements in Stem Cell Engineering: Inducing Tissue-Specific Differentiation
Advancements in stem cell engineering hold promise for overcoming existing challenges and enhancing the therapeutic potential of stem cell therapy in SCI. By modulating the expression of specific transcription factors or signaling pathways, researchers can induce tissue-specific differentiation of stem cells into desired cell lineages, such as neurons or glial cells, optimizing their functional integration within the injured spinal cord. Furthermore, advances in gene editing technologies, such as CRISPR-Cas9, offer precise tools for correcting genetic mutations associated with neurological disorders, paving the way for personalized medicine approaches in regenerative medicine.
Personalized Medicine Approaches: Tailoring Therapies to Individual Patients
Personalized medicine approaches aim to tailor therapeutic interventions to the unique characteristics and needs of individual patients, optimizing treatment outcomes and minimizing adverse effects. In the context of SCI, personalized medicine strategies encompass patient-specific stem cell sources, delivery methods, and adjunctive therapies based on genetic, immunological, and neuroimaging profiles. Biomarker-based approaches enable early identification of patients likely to benefit from stem cell therapy, facilitating targeted interventions and optimizing resource allocation in healthcare settings.
Addressing Immunological Responses and Host-Tissue Integration
Immunological responses and host-tissue integration represent significant challenges in stem cell therapy for SCI, necessitating innovative strategies to enhance immune tolerance and promote functional recovery. Immunomodulatory approaches, such as co-transplantation of immunosuppressive cells or engineering stem cells to evade immune detection, mitigate the risk of rejection and enhance long-term engraftment within the host tissue. Furthermore, bioengineering strategies, including biomaterial scaffolds and growth factor delivery systems, create a supportive microenvironment for stem cell survival, proliferation, and differentiation, enhancing their therapeutic efficacy in SCI.
Cost-Benefit Analysis and Accessibility
Economic Impact of Stem Cell Therapy: Assessing Healthcare Costs and Societal Benefits
The economic impact of stem cell therapy in SCI extends beyond direct healthcare costs to encompass broader societal benefits, including improvements in patient outcomes, quality of life, and productivity. While initial investments in stem cell research and clinical translation may be substantial, the long-term economic benefits, such as reduced healthcare expenditures, decreased disability rates, and increased workforce participation, justify the financial investment in regenerative medicine technologies. Furthermore, cost-effectiveness analyses provide valuable insights into the economic viability of stem cell therapy compared to conventional treatment modalities, informing healthcare decision-making and resource allocation.
Accessibility Issues: Disparities in Access to Innovative Treatments
Despite the potential benefits of stem cell therapy, disparities in access to innovative treatments pose challenges to equitable healthcare delivery and patient outcomes. Socioeconomic factors, geographical location, and healthcare infrastructure influence access to stem cell therapies, creating barriers for marginalized populations and underserved communities. Addressing these disparities requires collaborative efforts between policymakers, healthcare providers, researchers, and patient advocacy groups to develop inclusive healthcare policies, expand healthcare coverage, and promote equitable distribution of innovative treatments, ensuring that all individuals have access to the benefits of regenerative medicine.
Health Policy Considerations: Financing and Insurance Coverage for Stem Cell Interventions
Health policy considerations play a critical role in shaping the adoption and implementation of stem cell therapy in clinical practice. Financing mechanisms, such as public funding, private investment, and philanthropic support, facilitate research and development efforts and promote innovation in regenerative medicine. Insurance coverage for stem cell interventions varies across jurisdictions, with some healthcare systems providing reimbursement for approved therapies, while others require patients to bear the financial burden of treatment. Advocacy efforts aimed at advocating for comprehensive insurance coverage and reimbursement policies for stem cell therapies are essential for ensuring equitable access and affordability for patients with SCI.
Global Collaborations and Knowledge Exchange
International Research Consortia: Sharing Data and Best Practices
International research consortia play a pivotal role in fostering collaboration and knowledge exchange among scientists, clinicians, and policymakers worldwide. By sharing data, resources, and best practices, these collaborative networks facilitate interdisciplinary research endeavors, accelerate scientific discovery, and advance the development of stem cell therapies for SCI. Furthermore, international collaborations enable researchers to access diverse patient populations, genetic backgrounds, and healthcare systems, enhancing the generalizability and translatability of research findings across different contexts.
Multidisciplinary Collaboration: Integrating Expertise Across Fields
Multidisciplinary collaboration is essential for addressing the complex challenges inherent in stem cell therapy for SCI and maximizing its therapeutic potential. By integrating expertise from diverse fields, including neuroscience, stem cell biology, bioengineering, and health policy, researchers can develop comprehensive solutions that encompass the entire spectrum of SCI management, from basic science research to clinical translation and healthcare delivery. Furthermore, collaboration between academia, industry, and government agencies facilitates technology transfer, commercialization, and regulatory approval of stem cell-based interventions, driving innovation and improving patient outcomes in SCI.
Open Access Initiatives: Facilitating Information Dissemination and Collaboration
Open access initiatives play a crucial role in promoting transparency, accessibility, and collaboration in stem cell research and clinical practice. By providing unrestricted access to scientific publications, datasets, and research tools, open access platforms facilitate information dissemination and knowledge sharing among researchers, clinicians, and patients worldwide. Furthermore, open access initiatives promote reproducibility and scientific rigor by enabling independent verification of research findings and fostering collaboration between research groups with diverse expertise and perspectives.
Patient Advocacy and Support Networks
Empowering Patients: Access to Information and Resources
Patient advocacy organizations play a vital role in empowering individuals with SCI by providing access to information, resources, and support networks. These organizations advocate for the needs and rights of patients with SCI, raise awareness about the impact of the condition on individuals and their families, and promote research funding and policy initiatives to advance treatments and improve quality of life. Furthermore, patient advocacy groups offer valuable peer support, education, and outreach programs, fostering resilience, empowerment, and community engagement among individuals affected by SCI.
Advocacy Organizations: Amplifying Patient Voices in Research and Policy
Advocacy organizations serve as powerful advocates for patients with SCI, amplifying their voices in research, policy, and healthcare decision-making processes. By engaging policymakers, healthcare providers, and the public, advocacy organizations raise awareness about the unmet needs and challenges faced by individuals with SCI and advocate for policy reforms to improve access to innovative treatments, rehabilitation services, and supportive care. Furthermore, advocacy groups mobilize grassroots efforts, organize fundraising events, and lobby for legislative initiatives to promote inclusivity, equity, and social justice for individuals living with SCI.
Peer Support Networks: Fostering Community and Encouragement
Peer support networks provide invaluable emotional, practical, and social support to individuals with SCI and their families, fostering a sense of belonging, connection, and empowerment. By connecting individuals with shared experiences and challenges, peer support networks offer a safe space for individuals to share their stories, exchange information, and seek guidance from others who have navigated similar experiences. Furthermore, peer mentors and support groups provide encouragement, motivation, and hope for individuals facing the uncertainties and complexities of living with SCI, promoting resilience, self-efficacy, and well-being.
Make an informed Decision
Stem cell therapy holds tremendous promise for addressing the unmet medical needs of individuals with SCI by promoting tissue repair, functional recovery, and quality of life. Despite the challenges and complexities inherent in translating stem cell research from bench to bedside, ongoing advancements in stem cell biology, bioengineering, and clinical translation offer new opportunities for innovation and progress in regenerative medicine. By leveraging multidisciplinary collaboration, international partnerships, and patient advocacy efforts, we can accelerate the development and implementation of stem cell therapies for SCI, paving the way for a future where individuals with spinal cord injuries can regain independence, mobility, and hope. As we embark on this journey towards a brighter future, let us heed the call to action and work together to harness the transformative potential of stem cells in healing the spinal cord and restoring hope to millions of individuals worldwide.